Label: CALAMINE- calamine 8% and zinc oxide 8% lotion
- NDC Code(s): 82645-900-96
- Packager: Pharma Nobis, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 22, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
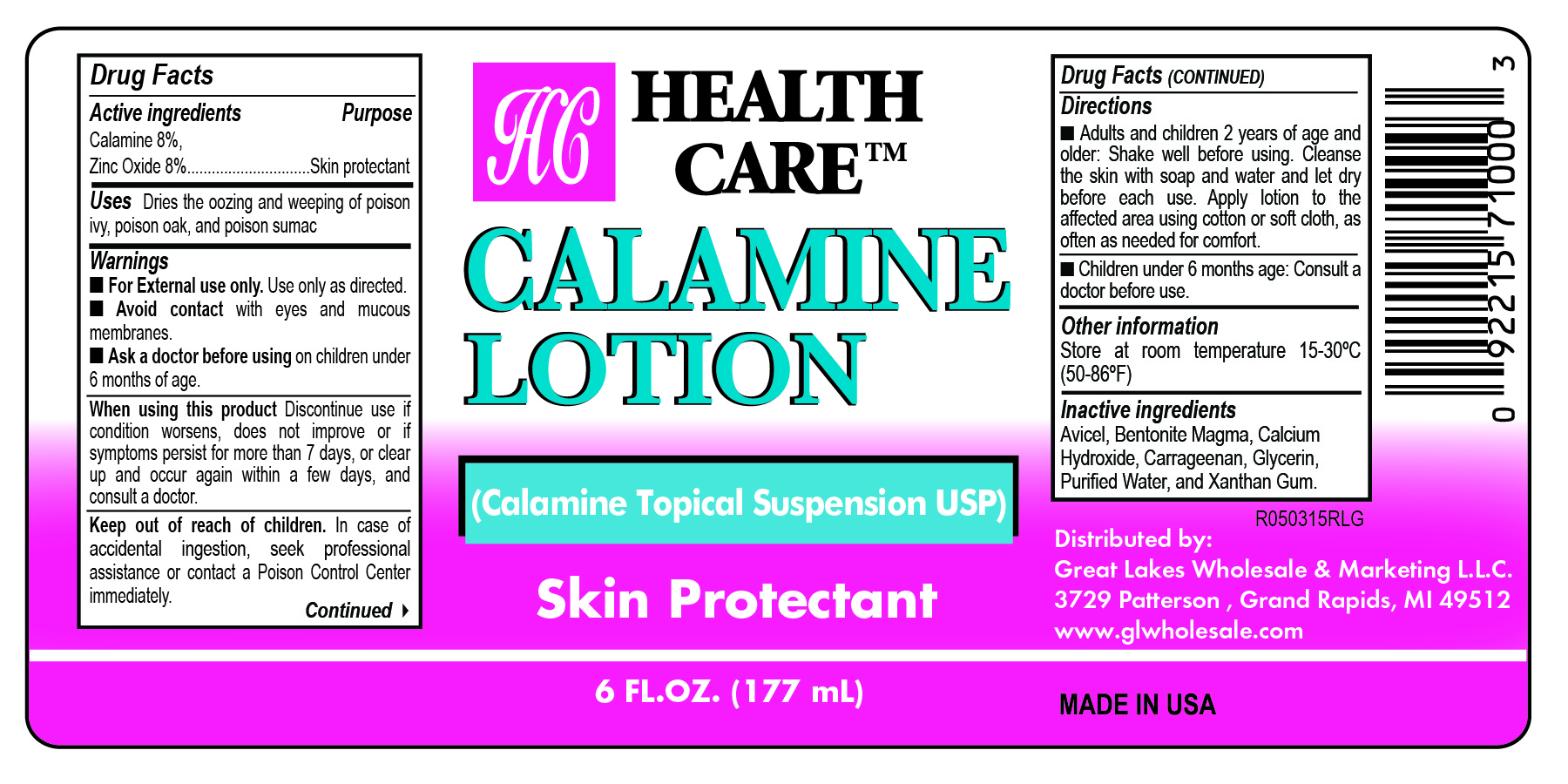
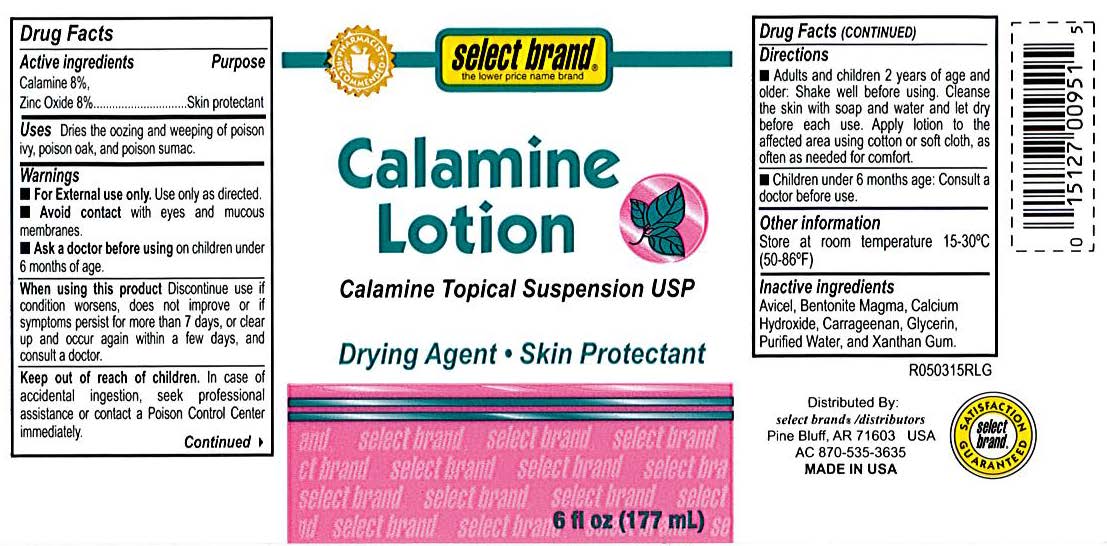
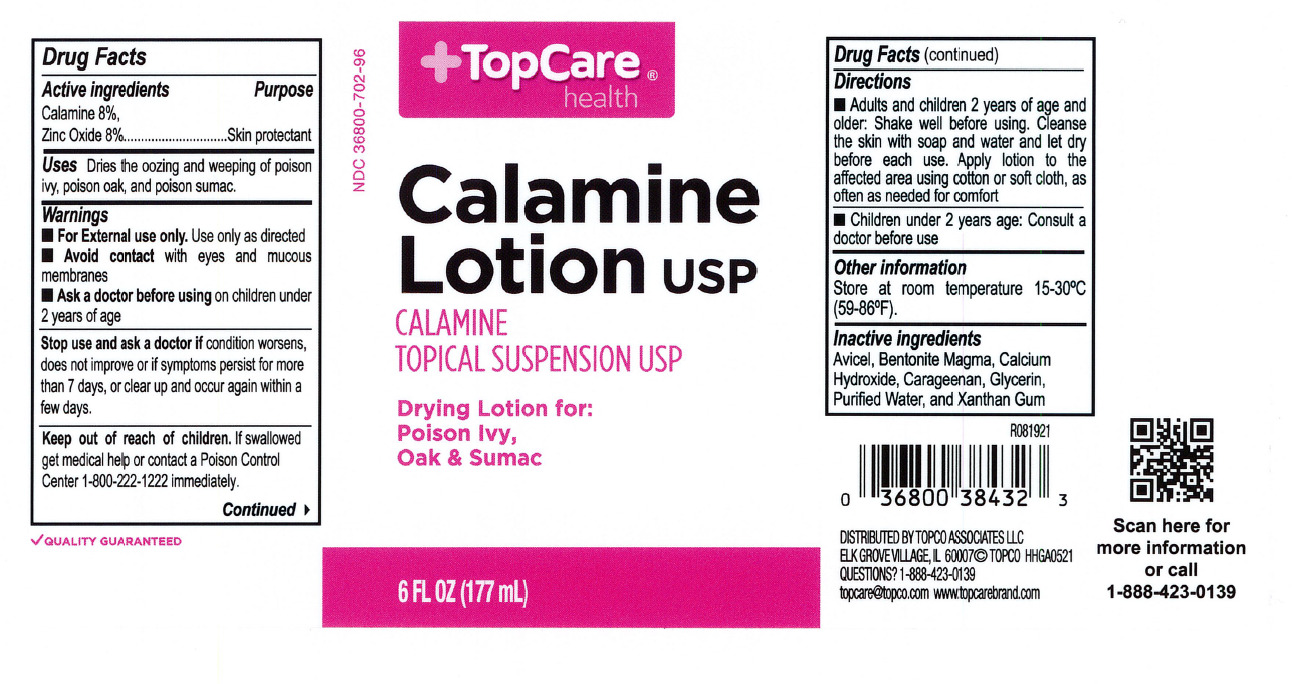
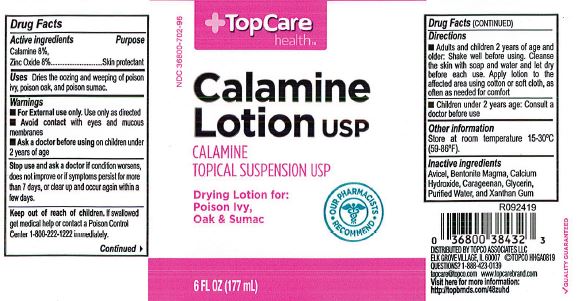
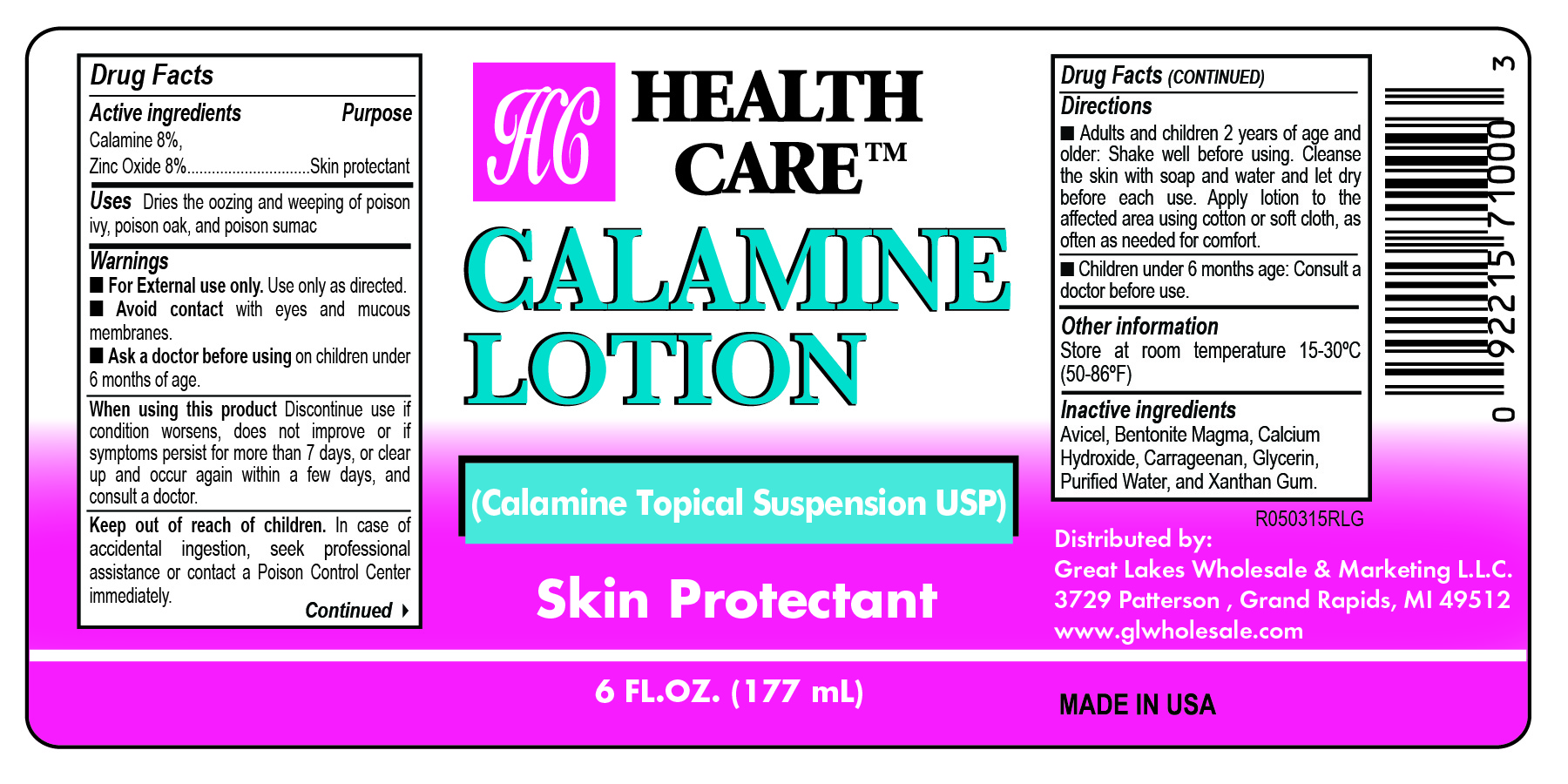
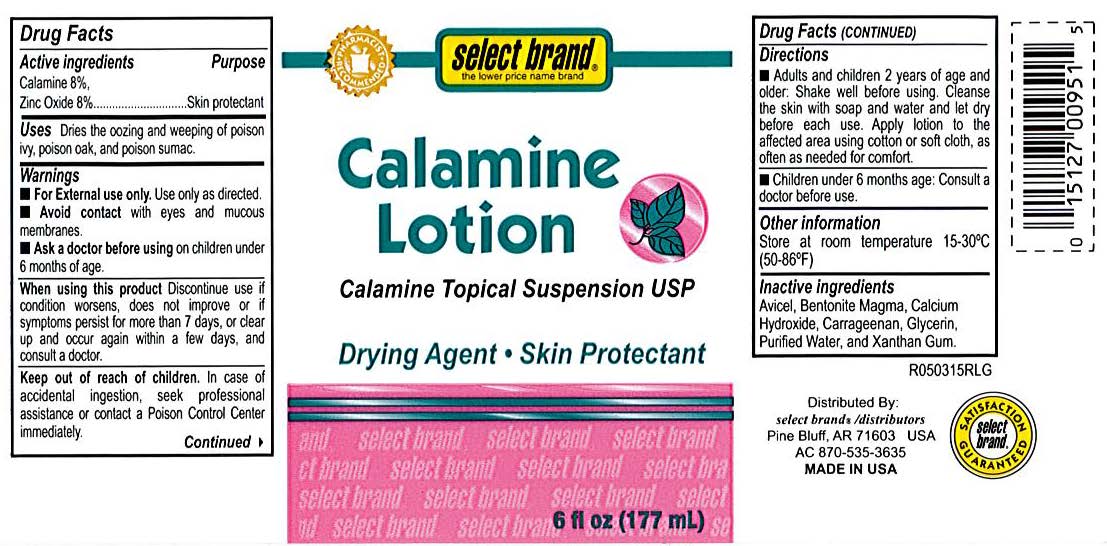
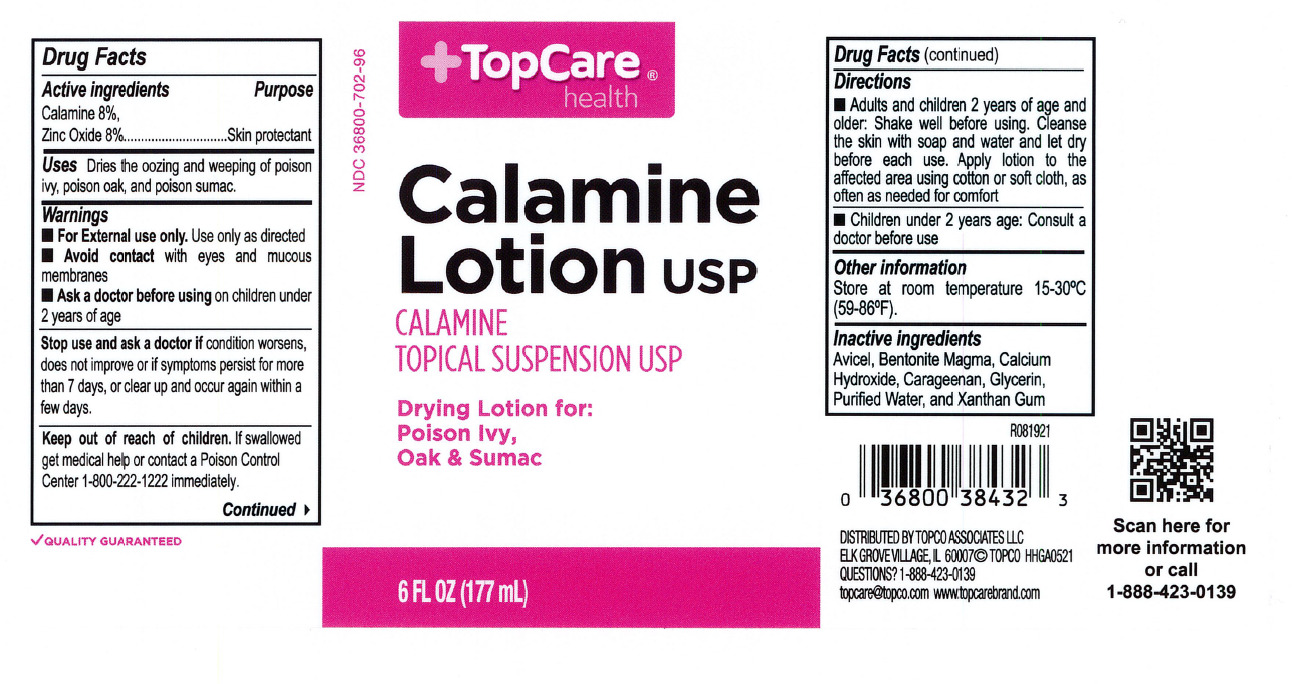
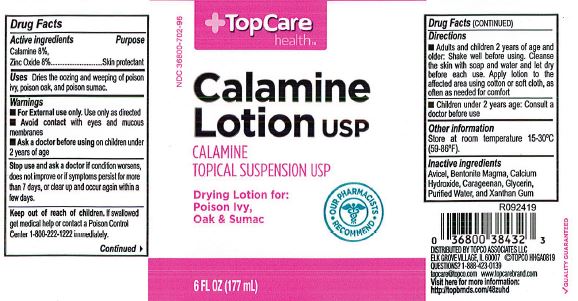
- Active Ingredients
- Purpose
- Uses
- Warnings
- When using this product
- Keep out of reach of children
- Directions
- Other information
- Inactive ingredients
- Good Neighbor Label
- Health Care Label
- Quality Choice Label
- Select Brand Label
- Sun Mark Label
- Premier Value
- Fred's Calamine
- DDM Label
- Rite Aid Label
- Harris Teeter Label
- Health Mart Label
- Dollar Tree Label
- Equate Label
- 99 Cent Label
- Top Care Label
- Assured Label
-
INGREDIENTS AND APPEARANCE
CALAMINE
calamine 8% and zinc oxide 8% lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82645-900 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 160 mg in 1 mL Inactive Ingredients Ingredient Name Strength CARRAGEENAN (UNII: 5C69YCD2YJ) XANTHAN GUM (UNII: TTV12P4NEE) CALCIUM HYDROXIDE (UNII: PF5DZW74VN) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) BENTONITE (UNII: A3N5ZCN45C) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82645-900-96 177 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/13/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 03/25/1998 Labeler - Pharma Nobis, LLC (118564114) Registrant - Pharma Nobis, LLC (118564114) Establishment Name Address ID/FEI Business Operations Pharma Nobis, LLC 118564114 label(82645-900) , manufacture(82645-900) , analysis(82645-900) , pack(82645-900)