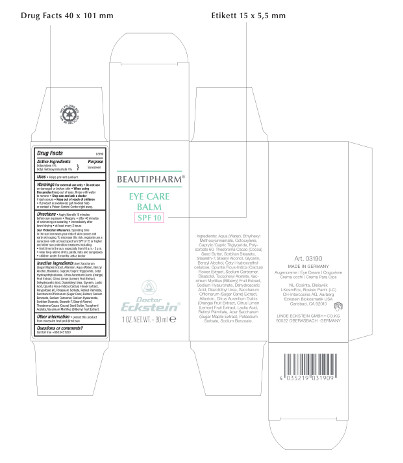
Label: BEAUTIPHARM EYE CARE BALM SPF 10- sunscreen cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 6889-0319-01, 6889-0319-02 - Packager: Linde Eckstein GmbH + Co. KG
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 5, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Beautipharm® Eye Care Balm
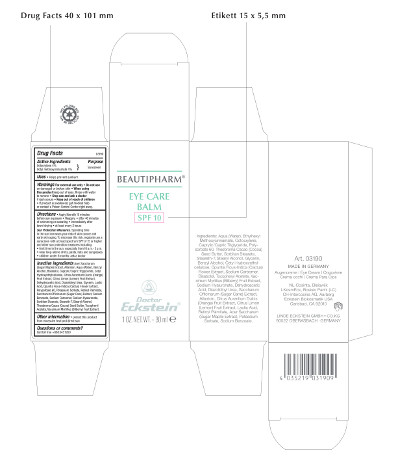
SPF 10
1 OZ. NET.WT. – 30 ml
Art. 03190
MADE IN GERMANY
Augencreme - Eye Cream – Oogcrème – Crema occhi – Crema Para Ojos
Ingredients: Aqua (Water), Ethylhexyl Methoxycinnamate, Octocrylene, Caprylic/Capric Triglyceride, Polysorbate 60, Theobroma Cacao (Cocoa) Seed Butter, Sorbitan Stearate, Steareth-7, Stearyl Alcohol, Glycerin, Benzyl Alcohol, Cetyl Hydroxyethylcellulose, Opuntia Ficus-Indica (Cactus) Flower Extract, Sodium Carbomer, Bisabolol, Tocopheryl Acetate, Vaccinium Myrtillus (Bilberry) Fruit Extract, Sodium Hyaluronate, Dehydroacetic Acid, Diazolidinyl Urea, Saccharum Officinarum (Sugar Cane) Extract, Allantoin, Citrus Aurantium Dulcis (Orange) Fruit Extract, Citrus Limon (Lemon) Fruit Extract, Lactic Acid, Retinyl Palmitate, Acer Saccharum (Sugar Maple) Extract, Potassium Sorbate, Sodium Benzoate.
NL-Cosinta, Bleiswijk
KosmEck Italia – Bosisio Parini (LC)
CH-Intercosina AG, Aarberg
Eckstein Biokosmetik USA
Carlsbad, CA 92010
LINDE ECKSTEIN GMBH+CO.KG
90522 OBERASBACH · GERMANY
Drug Facts
Active Ingredients
Octocrylene 4%
Octyl methoxycinnamate 4%
Purpose
Sunscreen
Uses
• Helps prevent sunburn
Warnings For external use only
• Do not use on damaged or broken skin
• When using this product keep out of eyes. Rinse with water to remove
• Stop use and ask a doctor if rash occurs
• Keep out of reach of children
• If product is swallowed, get medical help or contact a Poison Control Center right away.
Directions
• Apply liberally 15 minutes before sun exposure
• Reapply:
• after 40 minutes of swimming or sweating
• immediately after towel drying
• at least every 2 hours
Sun Protection Measures.
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
• limit time in the sun, especially from 10 a.m. - 2 p.m.
• wear long-sleeve shirts, pants, hats and sunglasses
• children under 6 months: ask a doctor
Inactive ingredients
Acer Saccharum (Sugar Maple) Extract, Allantoin, Aqua (Water), Benzyl Alcohol, Bisabolol, Caprylic/Capric Triglyceride, Cetyl Hydroxyethylcellulose, Citrus Aurantium Dulcis (Orange) Fruit Extract, Citrus Limon (Lemon) Fruit Extract, Dehydroacetic Acid, Diazolidinyl Urea, Glycerin, Lactic Acid, Opuntia Ficus-Indica (Cactus) Flower Extract, Polysorbate 60, Potassium Sorbate, Retinyl Palmitate, Saccharum Officinarum (Sugar Cane) Extract, Sodium Benzoate, Sodium Carbomer, Sodium Hyaluronate, Sorbitan Stearate, Steareth-7, Stearyl Alcohol, Theobroma Cacao (Cocoa) Seed Butter, Tocopheryl Acetate, Vaccinium Myrtillus (Bilberry) Fruit Extract.
Other information
• protect this product from excessive heat and direct sun
Questions or comments?
Call toll free +888 345 1950
-
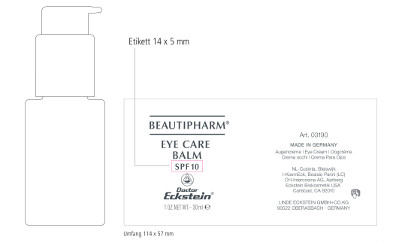
Beautipharm® Eye Care Balm
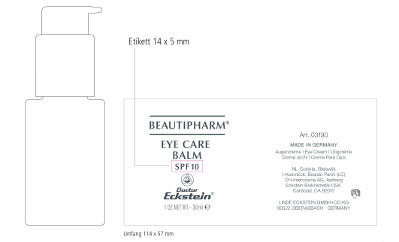
SPF10
1 OZ.NET.WT.– 30 ml
Art. 03190
MADE IN GERMANY
Augencreme - Eye Cream – Oogcrème – Crema occhi – Crema Para Ojos
NL-Cosinta, Bleiswijk
KosmEck Italia – Bosisio Parini (LC)
CH-Intercosina AG, Aarberg
Eckstein Biokosmetik USA
Carlsbad, CA 92010
LINDE ECKSTEIN GMBH+CO.KG
90522 OBERASBACH · GERMANY
-
INGREDIENTS AND APPEARANCE
BEAUTIPHARM EYE CARE BALM SPF 10
sunscreen creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:6889-0319 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 1.33 g in 30 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1.33 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 9.335 g in 30 mL MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) 3.33 g in 30 mL POLYSORBATE 60 (UNII: CAL22UVI4M) 3.33 g in 30 mL COCOA BUTTER (UNII: 512OYT1CRR) 3.33 g in 30 mL SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) 3.33 g in 30 mL STEARETH-7 (UNII: 820H8P0BYX) 1 g in 30 mL STEARYL ALCOHOL (UNII: 2KR89I4H1Y) 1 g in 30 mL GLYCERIN (UNII: PDC6A3C0OX) 1 g in 30 mL BENZYL ALCOHOL (UNII: LKG8494WBH) 0.3 g in 30 mL CETYL HYDROXYETHYLCELLULOSE (350000 MW) (UNII: T7SWE4S2TT) 0.3 g in 30 mL OPUNTIA FICUS-INDICA FLOWER (UNII: 83YSP51SMA) 0.3 g in 30 mL CARBOMER HOMOPOLYMER TYPE C (UNII: 4Q93RCW27E) 0.16 g in 30 mL LEVOMENOL (UNII: 24WE03BX2T) 0.1 g in 30 mL .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) 0.1 g in 30 mL BILBERRY (UNII: 9P2U39H18W) 0.1 g in 30 mL HYALURONATE SODIUM (UNII: YSE9PPT4TH) 0.1 g in 30 mL DEHYDROACETIC ACID (UNII: 2KAG279R6R) 0.09 g in 30 mL DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) 0.03 g in 30 mL SUGARCANE (UNII: 81H2R5AOH3) 0.03 g in 30 mL ALLANTOIN (UNII: 344S277G0Z) 0.03 g in 30 mL ORANGE (UNII: 5EVU04N5QU) 0.01 g in 30 mL LEMON (UNII: 24RS0A988O) 0.01 g in 30 mL LACTIC ACID (UNII: 33X04XA5AT) 0.01 g in 30 mL VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) 0.01 g in 30 mL ACER SACCHARUM BARK/SAP (UNII: Z120VL0KAC) 0.003 g in 30 mL POTASSIUM SORBATE (UNII: 1VPU26JZZ4) 0.001 g in 30 mL SODIUM BENZOATE (UNII: OJ245FE5EU) 0.001 g in 30 mL Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:6889-0319-02 1 in 1 BOX 11/24/2016 1 NDC:6889-0319-01 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 06/30/2014 Labeler - Linde Eckstein GmbH + Co. KG (316104744) Registrant - Linde Eckstein GmbH + Co. KG (316104744) Establishment Name Address ID/FEI Business Operations Linde Eckstein GmbH + Co. KG 316104744 manufacture(6889-0319)