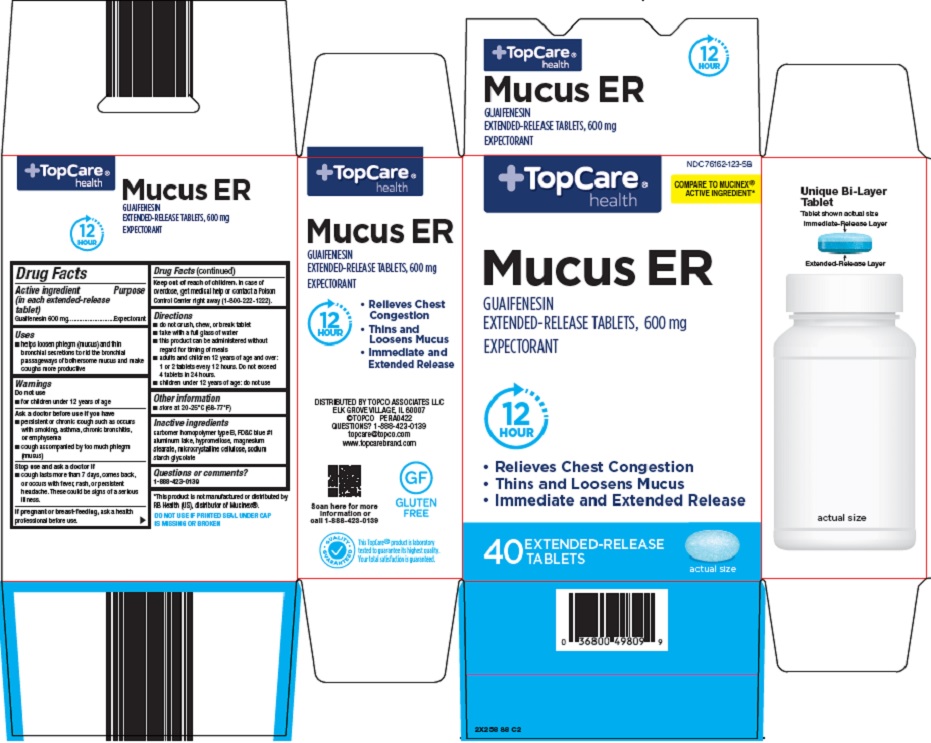
Label: TOPCARE MUCUS ER- guaifenesin tablet, multilayer, extended release
- NDC Code(s): 76162-123-58, 76162-123-60
- Packager: Topco Associates LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredient (in each extended-release tablet) Guaifenesin 600 mg
-
Purpose Expectorant
-
Uses • helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
-
Warnings Do not use - • for children under 12 years of age - Ask a doctor before use if you have - • persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or ...
-
Directions • do not crush, chew, or break tablet - • take with a full glass of water - • this product can be administered without regard for the timing of meals - • adults and children 12 years of age and over: 1 ...
-
Other information • store at 20-25°C (68-77°F)
-
Inactive ingredients carbomer homopolymer type B, FD&C blue #1 aluminum lake, hypromellose, magnesium stearate, microcrystalline cellulose, sodium starch glycolate
-
Questions or comments? 1-888-423-0139
-
Package/Label Principal Display Panel TopCare® health - COMPARE TO MUCINEX® ACTIVE INGREDIENT - Mucus ER - GUAIFENESIN - EXTENDED-RELEASE TABLETS, 600 mg - EXPECTORANT - 12 HOUR - • Relieves Chest Congestion - • Thins and Loosens Mucus - • Immediate ...
-
INGREDIENTS AND APPEARANCEProduct Information