Label: ITRAFUNGOL- itraconazole injection, solution
- NDC Code(s): 51311-148-52
- Packager: Virbac AH, Inc
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated September 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- GENERAL PRECAUTIONS
- Description
- Indication
-
Dosage and Administration
The solution should be administered orally using the enclosed graduated dosing syringe.
The daily dosage is 5 mg/kg (0.5 mL/kg) body weight administered once daily on alternating weeks for 3 treatment cycles. Cats are treated during weeks 1, 3, and 5, and left untreated during weeks 2 and 4.
7 days 7 days 7 days 7 days 7 days Daily treatment No treatment Daily treatment No treatment Daily treatment Each line on the dosing syringe represents 0.05 mL of oral solution.
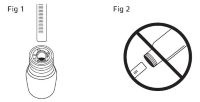
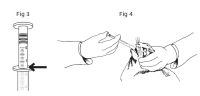
Table 1: Dose Table for ITRAFUNGOL Weight of Cat Volume of ITRAFUNGOL 0.5 lb 0.1 mL 1.0 lb 0.2 mL 1.5 lb 0.35 mL 2.0 lb 0.45 mL 2.5 lb 0.55 mL 3.0 lb 0.7 mL 3.5 lb 0.8 mL 4.0 lb 0.9 mL 4.5 lb 1.0 mL 5.0 lb 1.15 mL 6.0 lb 1.35 mL 7.0 lb 1.6 mL 8.0 lb 1.8 mL 9.0 lb 2.0 mL 10.0 lb 2.25 mL 11.0 lb 2.5 mL 12.0 lb 2.7 mL 13.0 lb 3.0 mL The solution should be administered orally using the enclosed graduated dosing syringe. Keep the bottle upright and insert the dosing syringe through the opening of the top of the bottle (Figure 1). Do not invert the bottle (Figure 2). Fill the syringe by pulling the plunger until it reaches the graduation corresponding to the correct mL dose as indicated at the top of the syringe ring (Figure 3). Treat the cat by slowly and gently administering the liquid into the mouth, allowing the cat to swallow the product (Figure 4). For cats weighing more than 13.0 lbs, the total dose will need to be calculated and given over two doses as the dosing syringe only holds 3.0 mL of solution.
After dosing, do not replace syringe in the bottle. Rinse and dry the syringe. The bottle cap should be screwed back on tightly.
- Contraindications
-
Warnings
User Safety Warnings
Not for use in humans. Keep this and all medications out of reach of children. Wash hands and exposed skin after use. In case of accidental contact with eyes, rinse thoroughly with water. In case of pain or irritation, seek medical advice. In case of accidental ingestion, rinse mouth with water and seek medical advice.
Special precautions for person administering the veterinary product to the animal:Microsporum canis dermatophytosis is a zoonotic disease (a disease that can be transmitted from animals to humans); therefore consult a physician if a suspected lesion occurs on a human. Wear protective gloves when handling the animal during treatment or when cleaning the syringe. Wash hands and exposed skin after handling the animal.
ITRAFUNGOL (itraconazole oral solution) has not been shown to be sporicidal; therefore in order to reduce zoonotic potential, environmental contamination, and to decrease course of the disease, topical and environmental treatment should also be utilized.
Animal Safety Warnings
ITRAFUNGOL oral solution has not been shown to be safe in pregnant cats (see Animal Safety). ITRAFUNGOL oral solution should only be used in pregnant or lactating cats when the benefits outweigh the potential risks.
Keep ITRAFUNGOL oral solution in a secure location out of reach of dogs, cats, and other animals to prevent accidental ingestion or overdose.
-
Precautions
In the laboratory effectiveness study, adverse reactions related to exposure to ITRAFUNGOL oral solution were primarily related to the gastrointestinal tract. Two ITRAFUNGOL-treated cats experienced transient hypersalivation during the dosing period. Vomiting was observed in 5 ITRAFUNGOL-treated cats (12.5%) during the dosing period compared to four cats (10%) in the control group. Diarrhea was observed in 9 ITRAFUNGOL-treated cats (22.5%) during the dosing period as compared to 7 cats (17.5%) in the control group.
One ITRAFUNGOL-treated cat showed mild increases in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) at the end of the dosing period. No related clinical signs were observed, and these values returned to normal by the end of the follow-up period. One cat in the ITRAFUNGOL-treated group was noted to have lip erythema and lip induration once during the study. -
Adverse Reactions
Post-Approval Experience (2021)
The following adverse events are based on post-approval adverse drug experience reporting for ITRAFUNGOL (itraconazole oral solution). Not all adverse events are reported to FDA/CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data.
The following adverse events reported in cats are listed in decreasing order of reporting frequency:
Anorexia, emesis, elevated liver enzymes, lethargy, weight loss, icterus, elevated total bilirubin, and diarrhea.
Death (including euthanasia) has been reported. Some of these deaths were associated with the adverse events reported above. -
Post-Approval Experience (2021)
The following adverse events are based on post-approval adverse drug experience reporting for ITRAFUNGOL (itraconazole oral solution). Not all adverse events are reported to FDA/CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data.
The following adverse events reported in cats are listed in decreasing order of reporting frequency:
Anorexia, emesis, elevated liver enzymes, lethargy, weight loss, icterus, elevated total bilirubin, and diarrhea.
Death (including euthanasia) has been reported. Some of these deaths were associated with the adverse events reported above.
-
Contact Information
To report suspected adverse drug events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Virbac AH, Inc. at 1-800-338-3659 or us.virbac.com. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae
-
Clinical Pharmacology
The mode of action of itraconazole is based on its highly selective binding ability to fungal cytochrome p-450 iso-enzymes. This inhibits the synthesis of ergosterol and affects membrane-bound enzyme function and membrane permeability. This effect is irreversible and causes structural degeneration of the fungal organism.
Itraconazole was absorbed rapidly following oral administration of ITRAFUNGOL oral solution to laboratory cats. Compared to the fasted state, administration of ITRAFUNGOL oral solution with food results in slightly higher (1.3 fold) mean total itraconazole exposure (AUC), delay in median Tmax (Fed 4 hours vs. Fasted 2 hours) and significant decrease (approximately 0.55 fold) in maximum plasma concentration (Cmax). ITRAFUNGOL oral solution can be administered with or without food. Itraconazole oral solution binds extensively to plasma proteins (> 99%), and distributes well to tissues. More than 30 metabolites are formed. Hydroxy-itraconazole is the parent metabolite and has antifungal activity. Excretion is rapid and primarily via the feces.
In cats, a single oral dose of 5 mg/kg results in a Cmax of 0.525 μg/mL post dose at 2 hours (Tmax). The AUC0-24h is 5.09 µg·h/mL and the half-life in plasma is 12.1 hours. After repeated daily administration for seven days at 5 mg/kg/day, the Cmax is doubled (1.05 μg/mL), the AUC0-24h is increased 3-fold (15.4 µg·h/
mL) and the plasma half-life is increased to 36 hours.In the therapeutic treatment schedule, itraconazole is almost completely cleared from plasma after each wash-out period. The hydroxy-itraconazole remains near or below the quantification limit in feline plasma after a single dose of itraconazole oral solution at 5 mg/kg. However, after repeated daily doses of itraconazole oral solution at 5 mg/kg for one week, the hydroxy-itraconazole Cmax of 0.059 µg/mL was reached at 2 hours (Tmax). Itraconazole concentrations in cat’s hair vary; an increase occurs during treatment to a median value of 3.0 μg/g (mean 5.2 μg/g) at the end of the third dosing week and concentrations drop slowly to 1.5 μg/g (mean 1.9 μg/g) at 14 days after final dosing. Concentrations of hydroxy-itraconazole in hair are insignificant
-
Effectiveness
Laboratory Study
Effectiveness was demonstrated using ITRAFUNGOL oral solution in a masked, placebo controlled laboratory study. Eighty cats were experimentally infected with Microsporum canis and treated with either ITRAFUNGOL oral solution or sterile water (control product) for the proposed therapeutic treatment schedule followed by a 4-week follow-up period. No topical therapy was used during this study. A statistical difference (P =0.0003) in mycological cure rate (defined as two consecutive negative mycological cultures) was demonstrated between cats treated with ITRAFUNGOL oral solution (24/40 or 60%) versus control (1/40 or 2.5%). Ninety percent of ITRAFUNGOL-treated cats (36/40) achieved at least one negative culture by the end of the study. Improvement was seen in inoculation site erythema and skin thickening by Day 7 and in crusts and scales by Day 14. By the end of the study, 98% of ITRAFUNGOL-treated cats had complete resolution of all clinical lesions, compared to 15% in the control group.Wood’s lamp cure (defined as no fluorescence at the base and mid-shaft of the hair) in the ITRAFUNGOL-treated group (39/40 or 97.5%) was higher compared to the control group (6/40 or 15%). Itraconazole MICs indicative of susceptibility were obtained in M. canis isolates from the two cats unsuccessfully treated with ITRAFUNGOL oral solution.
Field Study
A masked, positive-controlled, multi-site field study was conducted in client-owned cats in Europe. In this study, 514 cats diagnosed with dermatophytosis were randomly administered itraconazole oral solution or an active control.Cats received a daily dose of either itraconazole oral solution for three alternating weeks plus a placebo tablet once daily for 5 consecutive weeks, or a placebo solution for three alternating weeks plus the active control once daily for five weeks. Success was evaluated on clinical cure, which was noted with a complete resolution of all clinical lesions. Four weeks after the end of treatment, 175 (83%) out of 207 cats treated with itraconazole oral solution were clinically cured.
Animal Safety
Margin of Safety Study with Recovery
In a margin of safety study, ITRAFUNGOL (itraconazole oral solution) was administered orally to 9-10 week old healthy kittens once daily at 0X (saline control), 1X (5 mg/kg), 3X (15 mg/kg), and 5X (25 mg/kg) the therapeutic dose for 17 alternating weeks (9 total weeks of dosing) followed by an 8 week recovery period. Hypersalivation during or immediately following dosing, vomiting, and loose stool were the most frequent abnormal clinical observations related to treatment with ITRAFUNGOL oral solution. Hypersalivation was limited to the 3X and 5X groups and was observed in every dosing cycle. Vomiting was noted at similar levels in the control, 1X and 3X groups; however, it occurred approximately twice as often in the 5X group. Mild gingival bleeding and perioral irritation (patchy alopecia and erythema) was noted in cats in the 3X and 5X groups. Food consumption was consistently higher throughout the study in the control group than the ITRAFUNGOL oral solution groups. The control group gained more weight during the study than the groups administered ITRAFUNGOL oral solution. Mild elevations in ALT were sporadically noted in all groups; however, the number of affected cats increased with the higher doses (two cats in the control group, two cats in the 1X group, three cats in the 3X group, and four cats in the 5X group). In most cats, ALT values peaked just above the upper limit of the reference range and were continuing to trend upward or were elevated yet stable at the end of the study. One cat in the 5X group exhibited inappetence progressing to anorexia, dehydration and vomiting during the first dosing cycle. This cat had repeated episodes of inappetence during the second and third dosing cycles. This cat also had markedly elevated ALT and AST values on Day 36 (693 U/L and 283 U/L, respectively), was treated with minimal supportive care and recovered to complete the study.
Margin of Safety StudyIn a margin of safety study, ITRAFUNGOL oral solution was administered orally to healthy adult cats once daily at 0X (saline control), 1X (5 mg/kg), 3X (15 mg/kg), and 5X (25 mg/kg) the therapeutic dose for 17 alternating weeks (9 total weeks of dosing) with no recovery period. Hypersalivation was the most frequent abnormal clinical observation related to treatment with ITRAFUNGOL oral solution and the incidence increased with the higher doses. One cat in group 4 (5X; Cat #26302) lost 22% of its body weight and had a number of episodes of vomiting, salivation, and anorexia during the treatment period. This cat also had renal lesions found on histopathology. Increasing trends were noted in ALT, AST, and creatinine values in some cats administered ITRAFUNGOL oral solution as compared to baseline values. Abnormal renal findings included proximal convoluted tubule acute degeneration in 3 cats in the 1X group and 3 cats in the 5X group; one 5X cat (cat #26302) also had proximal convoluted tubule marked pallor and focal mononuclear cell infiltration in the kidneys. In the lungs, one 3X group cat and five 5X cats had intra-alveolar foamy macrophages; five 5X group cats had intra-alveolar syncytial cells.
These histopathology findings are likely related to exposure to ITRAFUNGOL oral solution, specifically the vehicle component hydroxypropyl--cyclodextrin (HPCD). There were no corresponding adverse clinical effects noted on observation or on clinical pathology analysis. In addition, similar changes have been described in literature in other species exposed to HPCD and have been reported to be reversible.Reproductive Safety
In a study of 16 pregnant queens administered itraconazole oral solution at 5 mg/kg bodyweight for a total of 21 days (7 days on alternate weeks) during gestation or lactation, there was a high frequency of fetal resorption (partial and total), abnormal fetuses, and abnormal maternal behaviors. Confounding factors, such as infectious disease (Chlamydia psittaci) in some cats made it difficult to establish a definitive relationship between administration of itraconazole and the abnormal findings. However, the results of this study reveal potential reproductive safety risks and do not support the safe use of ITRAFUNGOL oral solution in pregnant queens.
- Storage conditions
- How supplied
- SPL UNCLASSIFIED SECTION
- Bottle label
-
INGREDIENTS AND APPEARANCE
ITRAFUNGOL
itraconazole injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:51311-148 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength itraconazole (UNII: 304NUG5GF4) (itraconazole - UNII:304NUG5GF4) itraconazole 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength Saccharin sodium (UNII: SB8ZUX40TY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51311-148-52 1 in 1 BOX 1 52 mL in 1 BOTTLE, GLASS Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141474 05/04/2022 Labeler - Virbac AH, Inc (131568396) Registrant - Virbac AH, Inc (131568396)