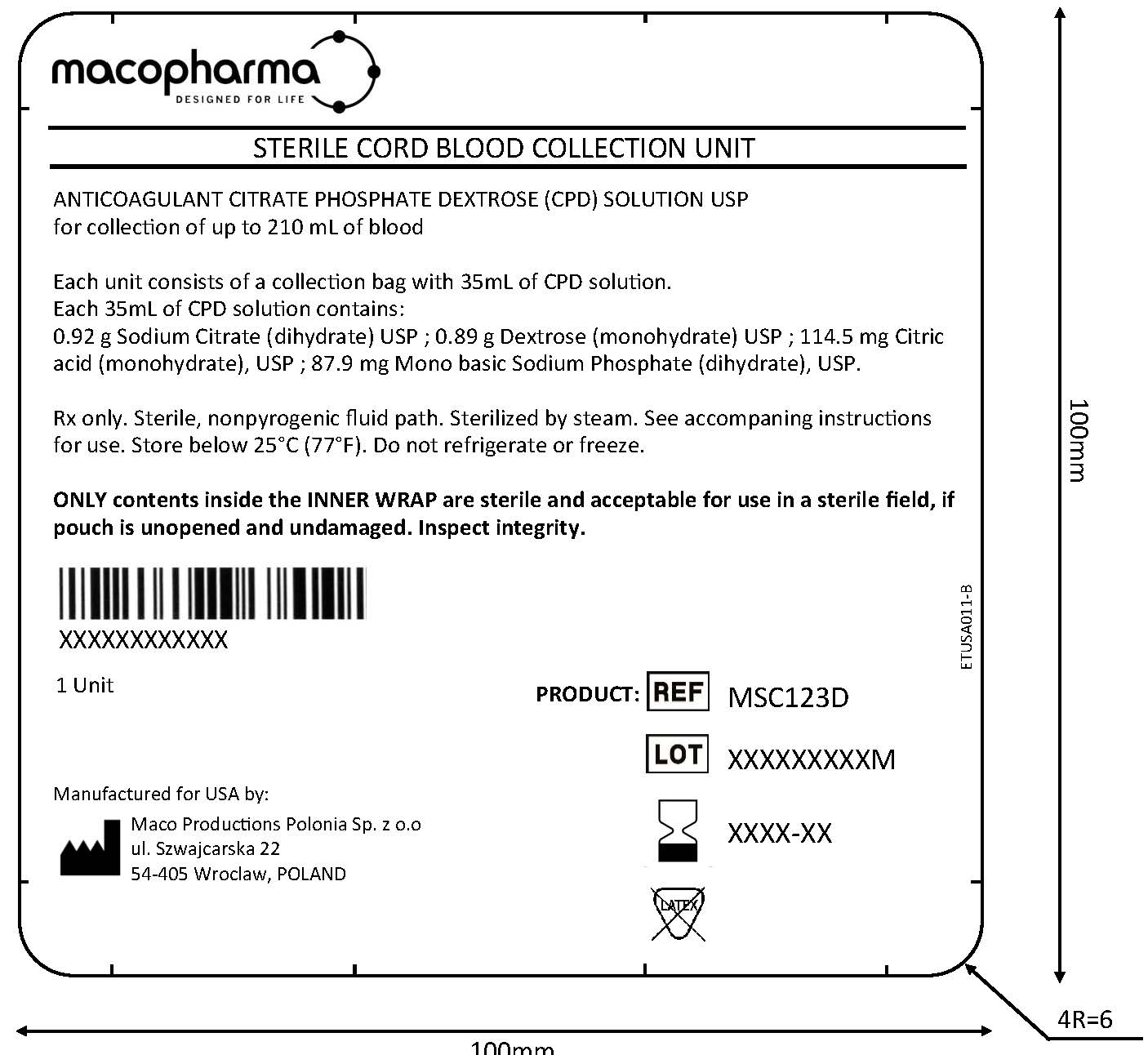
Label: STERILE CORD BLOOD COLLECTION UNIT (MSC123D)- anticoagulant citrate phosphate dextrose injection, solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 14498-005-01 - Packager: Maco Productions
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 6, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
MACO BIOTECH Collection- Sterile Cord Blood Collection Unit (MSC123D)
The MSC123D Sterile Cord Blood Collection Unit consists of 250 mL collection bag containing 35 mL of Anticoagulant Citrate Phosphate Dextrose Solution USP (CPD) and one 12 gauge needle with a protective shield (SECUVAM) for the used needle. Sterile, non-pyrogenic fluid path. Sterilized by steam.
Rx only.
This product is not made with natural rubber latex.
- INDICATIONS AND USAGE
- WARNINGS
-
PRECAUTIONS
- The kit within its aluminum outer overwrap must be stored at room temperature and protected from freezing: do not refrigerate or freeze.
- DO NOT use if the overwraps or bags show any signs of alteration.
- This is a single use sterile kit: under no circumstances should it be reused.
- For optimal cord blood quality it is recommended to maintain the cord blood at an ambient temperature (18-26°C / 64.4-78.8°F) or cold temperature (4-2°C / 39.2-53.6°F) and process within 48 hours of collection.1,2,3.
If the collection volume is less than 60 mL, the cord blood should be processed within 24 hours of collection.
- HOW SUPPLIED
-
INSTRUCTIONS FOR USE
Carefully Read All the Instructions Before Use
Representative Product Drawing
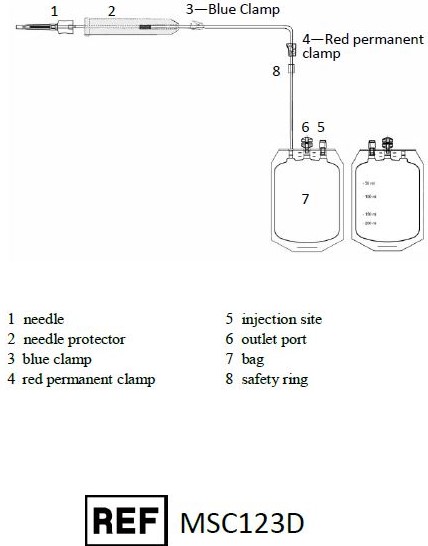
I. PREPARATION
1. Before use, verify the integrity of the collection unit (overwrap, outlet ports, tubing…).
2. Only contents inside the inner wrap are sterile and acceptable for use in a sterile field if pouch is unopened and undamaged.II. COLLECTION PROCEDURE
1. Close the clamp 3 (clamp 4 should remain open).
2. After clamping and sectioning of the umbilical cord, place the collection bag on a mixer and perform a venipuncture at lower end of the umbilical cord with needle 1. Note: Umbilical venipunctures can be done before or after the expulsion of the placenta according to your local procedures.
3. Open clamp 3.
4. Stimulate cord blood flow by massaging the cord from the top downward to increase volume of collection.
5. When collection is completed, close clamp 3.
6. Remove needle 1 and slide down SECUVAM 2 (needle protector) over needle 1.III. ADDITION OF CORD BLOOD FROM THE TUBE TO THE SAMPLING POUCH
1. Move clamp 3 at the top of the collection line close to the needle protector 2
2. Using a blood stripper, strip the tubing from the top of the tube towards the safety ring expelling the blood in the tubing into collection bag 7.
3. Repeat this operation 3 times.
4. Mix collection bag 7.
5. Close clamp 3.IV. END OF COLLECTION
1. Close clamp 4 to secure the collection above the safety ring 8.
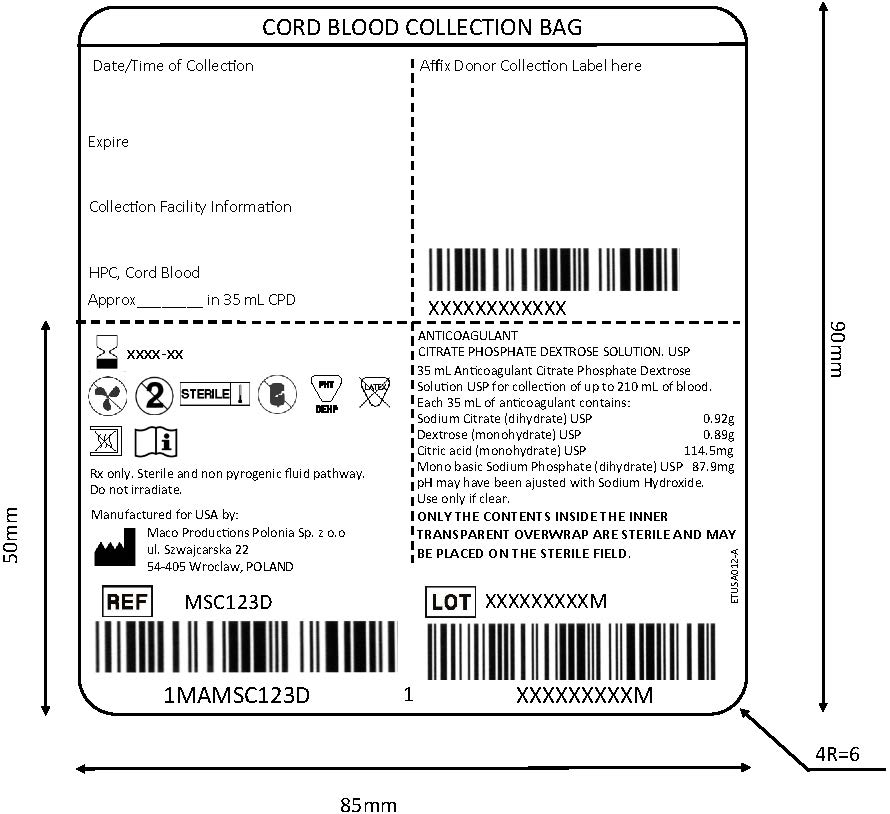
2. Seal the tubing above the clamp 4.
3. Fill in collection volume on the collection bag label.
4. For optimal cord blood quality, maintain the cord blood at an ambient temperature (18-26°C / 64.4-78.8°F) or cold temperature (4-12°C / 39.2-53.6°F) and process within 48 hours of collection.1,2,3If the collection volume is less than 60 mL, the cord blood should be processed within 24 hours of collection.
VI. CONTACT INFORMATION
Manufactured for United States of America (U.S.A.) by Maco Productions Polonia, Sp. z o.o. ul. Szwajcarska 22, 54-405 Wroclaw, POLAND +48 71 37 60 110
For consumer information contact:
Distributed by Uneklo USA, Inc., dba Macopharma USA, 3075 Breckinridge Boulevard, Suite 405, Duluth, GA 30096, USA (770) 270-6867VII. REFERENCES
1. Pope, B., Mitsakos, K., Bilgin, A., Hokin, B. and Grant, R: Predicting overall viability of cord blood harvests. Transfusion 2012; 52:1079-1085
2. Salge-Bartels U. Huber. M, Kleiner K, Volkers P, Seitz R, Heiden M: Evaluation of Quality Parameters for Cord Blood Donations, Transfus Med Hemother 2009; 36:317-324
3. Prof. Dr.Kogler, Jose Carreras CBB, medical Center, University of Duesseldorf : General information on the institution and the production facility of the Jose Carreras Cord Blood Bank. 2014 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
STERILE CORD BLOOD COLLECTION UNIT (MSC123D)
anticoagulant citrate phosphate dextrose injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:14498-005 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CITRATE (UNII: 1Q73Q2JULR) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) SODIUM CITRATE 0.92 g in 35 mL DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 0.89 g in 35 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 114.5 g in 35 mL SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE (UNII: 5QWK665956) (PHOSPHATE ION - UNII:NK08V8K8HR) SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE 87.9 g in 35 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14498-005-01 1 in 1 KIT 1 35 mL in 1 KIT; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN125552 10/02/2020 Labeler - Maco Productions (265492868)