Label: STOMASIN- gypsum fibrosum extract, forsythia viridissima fruit extract, arctium lappa fruit extract, platycodon grandiflorus root extract, saposhnikovia divaricata root extract, glycyrrhiza uralensis root extract, ostericum koreanum extract, schizonepeta tenuifolia extract capsule, coated
- NDC Code(s): 82871-301-01
- Packager: MedicareBio.Co.Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
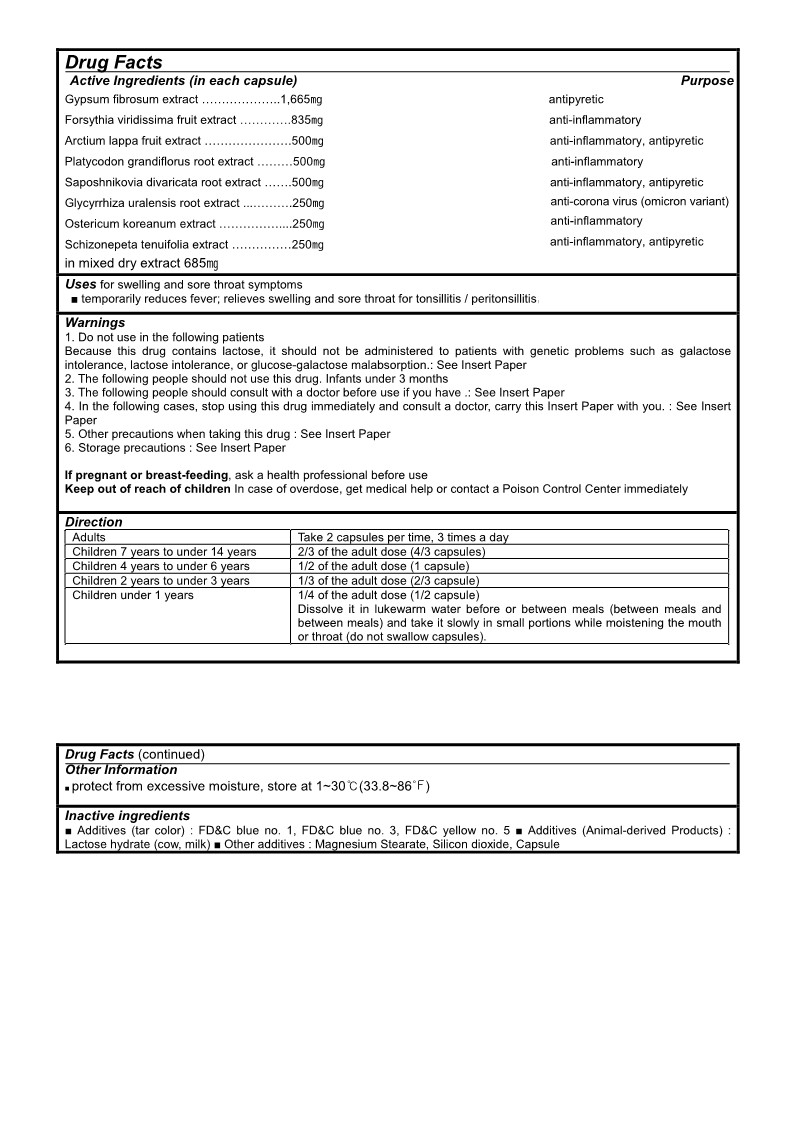
- Active Ingredients
- Purpose
- Uses
- Warnings
- Warnings
- Warnings
- Warnings
- Warnings
-
Directions
Adults Take 2 capsules per time, 3 times a day
Children 7 years to under 14 years 2/3 of the adult dose (4/3 capsules)
Children 4 years to under 6 years 1/2 of the adult dose (1 capsule)
Children 2 years to under 3 years 1/3 of the adult dose (2/3 capsule)
Children under 1 years 1/4 of the adult dose (1/2 capsule)
Dissolve it in lukewarm water before or between meals (between meals and between meals) and take it slowly in small portions while moistening the mouth or throat (do not swallow capsules). - Other Information
- Inactive Ingredients
- Display Panel
-
INGREDIENTS AND APPEARANCE
STOMASIN
gypsum fibrosum extract, forsythia viridissima fruit extract, arctium lappa fruit extract, platycodon grandiflorus root extract, saposhnikovia divaricata root extract, glycyrrhiza uralensis root extract, ostericum koreanum extract, schizonepeta tenuifolia extract capsule, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82871-301 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OSTERICUM GROSSESERRATUM ROOT (UNII: 9IGM98814N) (OSTERICUM GROSSESERRATUM ROOT - UNII:9IGM98814N) OSTERICUM GROSSESERRATUM ROOT 250 mg CALCIUM SULFATE, UNSPECIFIED FORM (UNII: WAT0DDB505) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM SULFATE, UNSPECIFIED FORM 1665 mg FORSYTHIA VIRIDISSIMA FRUIT (UNII: P4ZY00SWES) (FORSYTHIA VIRIDISSIMA FRUIT - UNII:P4ZY00SWES) FORSYTHIA VIRIDISSIMA FRUIT 835 mg ARCTIUM LAPPA FRUIT (UNII: EA541308MV) (ARCTIUM LAPPA FRUIT - UNII:EA541308MV) ARCTIUM LAPPA FRUIT 500 mg PLATYCODON GRANDIFLORUM ROOT (UNII: 2DF0NS0O2Z) (PLATYCODON GRANDIFLORUM ROOT - UNII:2DF0NS0O2Z) PLATYCODON GRANDIFLORUM ROOT 500 mg SAPOSHNIKOVIA DIVARICATA ROOT (UNII: 8H84LFK2QD) (SAPOSHNIKOVIA DIVARICATA ROOT - UNII:8H84LFK2QD) SAPOSHNIKOVIA DIVARICATA ROOT 500 mg NEPETA TENUIFOLIA FLOWERING TOP (UNII: 2FN3BA1MZE) (NEPETA TENUIFOLIA FLOWERING TOP - UNII:2FN3BA1MZE) NEPETA TENUIFOLIA FLOWERING TOP 250 mg GLYCYRRHIZA URALENSIS ROOT (UNII: 42B5YD8F0K) (GLYCYRRHIZA URALENSIS ROOT - UNII:42B5YD8F0K) GLYCYRRHIZA URALENSIS ROOT 250 mg Inactive Ingredients Ingredient Name Strength FD&C YELLOW NO. 5 (UNII: I753WB2F1M) MAGNESIUM STEARATE (UNII: 70097M6I30) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) Product Characteristics Color blue (blue and dark green capsule) Score score with uneven pieces Shape CAPSULE (brown powder encapsulated in blue and dark green opaque capsule) Size 22mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82871-301-01 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 07/13/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 07/13/2022 Labeler - MedicareBio.Co.Ltd (695630697) Registrant - MedicareBio.Co.Ltd (695630697) Establishment Name Address ID/FEI Business Operations MedicareBio.Co.Ltd 695630697 manufacture(82871-301)