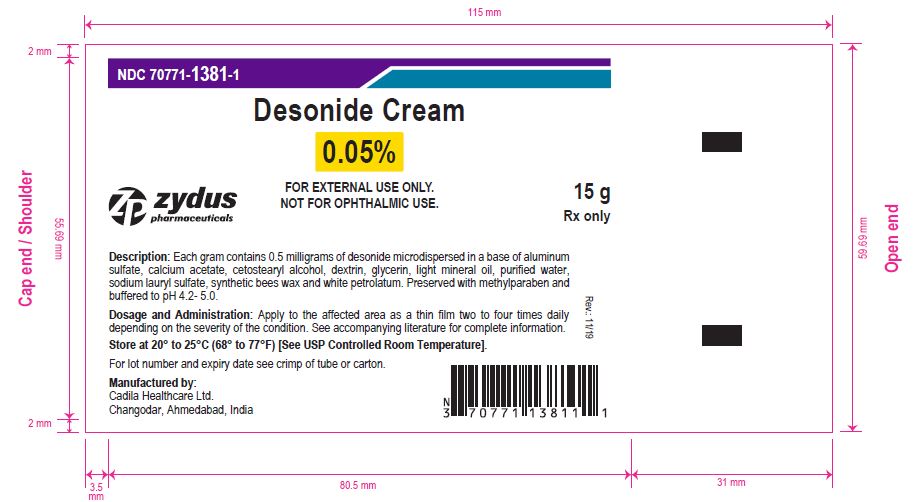
Label: DESONIDE cream
- NDC Code(s): 70771-1381-1, 70771-1381-3
- Packager: Zydus Lifesciences Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 5, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DESONIDE
desonide creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1381 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DESONIDE (UNII: J280872D1O) (DESONIDE - UNII:J280872D1O) DESONIDE 0.5 mg in 1 g Inactive Ingredients Ingredient Name Strength ALUMINUM SULFATE (UNII: 34S289N54E) CALCIUM ACETATE (UNII: Y882YXF34X) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) GLYCERIN (UNII: PDC6A3C0OX) ICODEXTRIN (UNII: 2NX48Z0A9G) LIGHT MINERAL OIL (UNII: N6K5787QVP) METHYLPARABEN (UNII: A2I8C7HI9T) PETROLATUM (UNII: 4T6H12BN9U) SODIUM LAURYL SULFATE (UNII: 368GB5141J) WATER (UNII: 059QF0KO0R) YELLOW WAX (UNII: 2ZA36H0S2V) Product Characteristics Color WHITE (WHITE TO OFF-WHITE) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1381-1 1 in 1 CARTON 11/25/2019 1 15 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:70771-1381-3 1 in 1 CARTON 11/25/2019 2 60 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210198 11/25/2019 Labeler - Zydus Lifesciences Limited (918596198) Registrant - Zydus Lifesciences Limited (918596198) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 650650802 ANALYSIS(70771-1381) , MANUFACTURE(70771-1381)