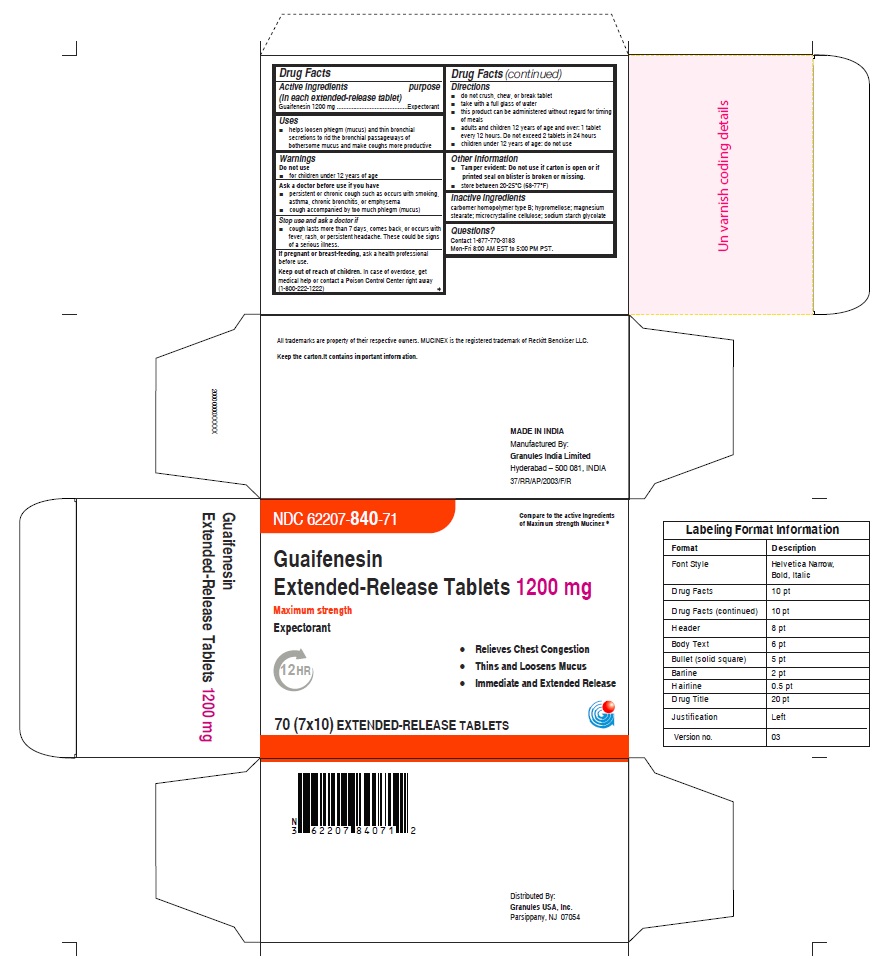
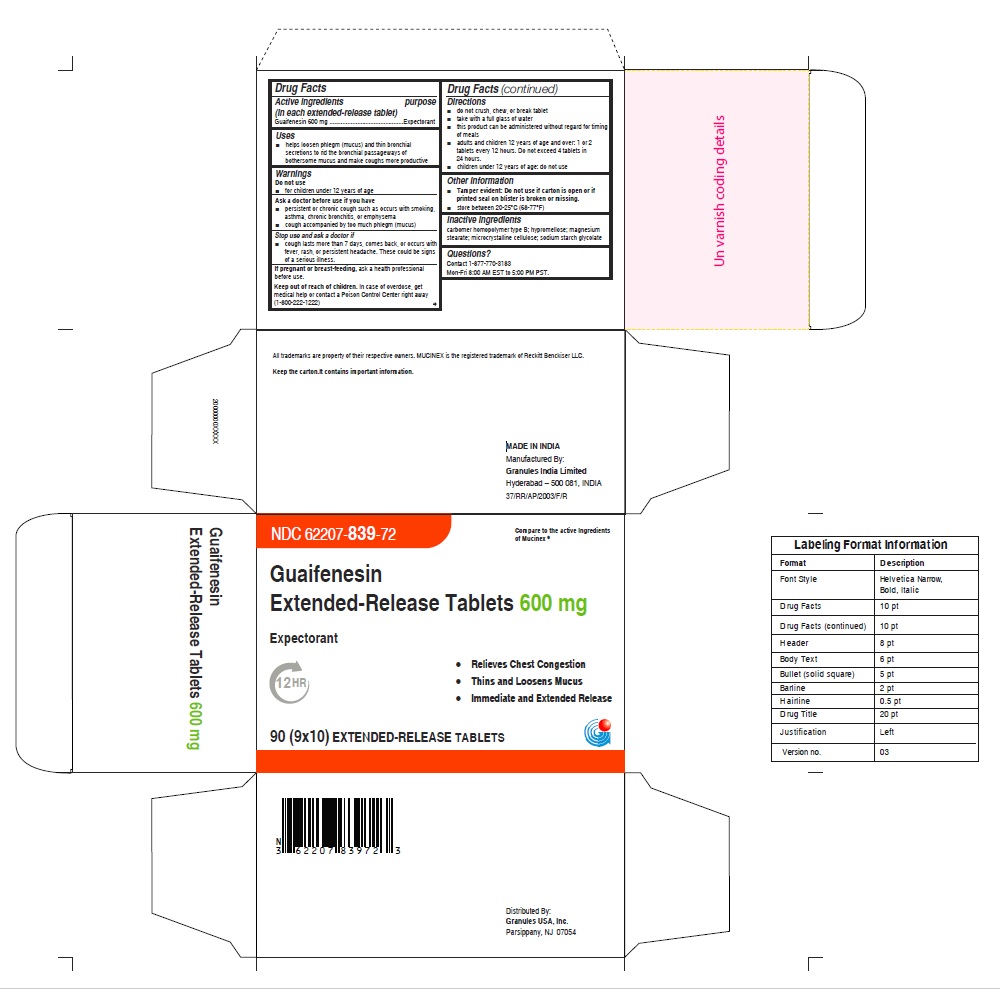
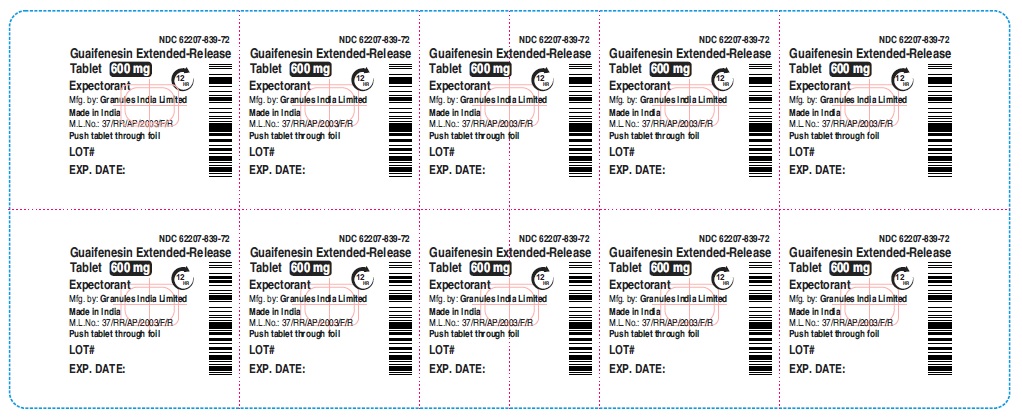
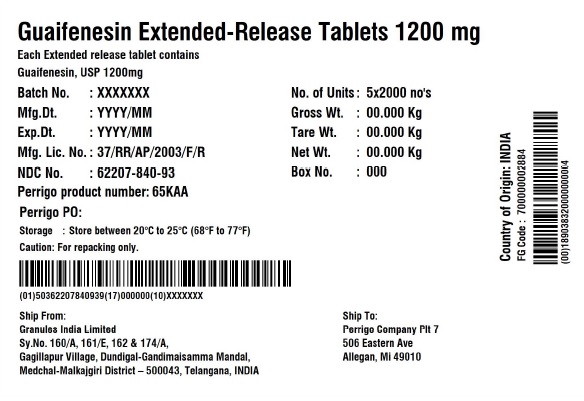
Label: GUAIFENESIN tablet, extended release
- NDC Code(s): 62207-839-72, 62207-840-71, 62207-840-93, 62207-840-99
- Packager: Granules India Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT(S)
- PURPOSE
- USE(S)
- WARNINGS
- ASK A DOCTOR BEFORE USE IF YOU HAVE
- STOP USE AND ASK DOCTOR IF
- PREGNANCY/BREASTFEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
- do not crush, chew, or break tablet
- take with a full glass of water
- this product can be administered without regard for timing of meals
- adults and children 12 years of age and over: 1 or 2 tablets every 12 hours. Do not exceed 4 tablets in 24 hours(For 600mg)
- adults and children 12 years of age and over: 1 tablet every 12 hours. Do not exceed 2 tablets in 24 hours.(For 1200mg)
- children under 12 years of age: do not use
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- QUESTIONS OR COMMENTS
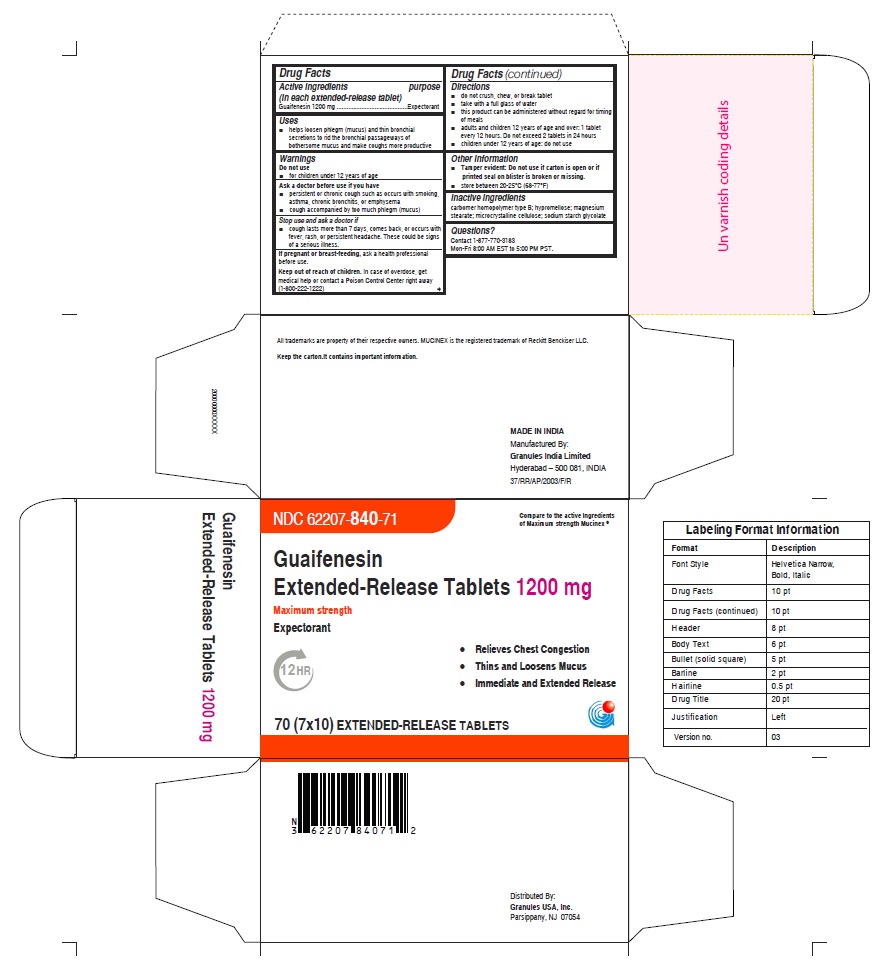
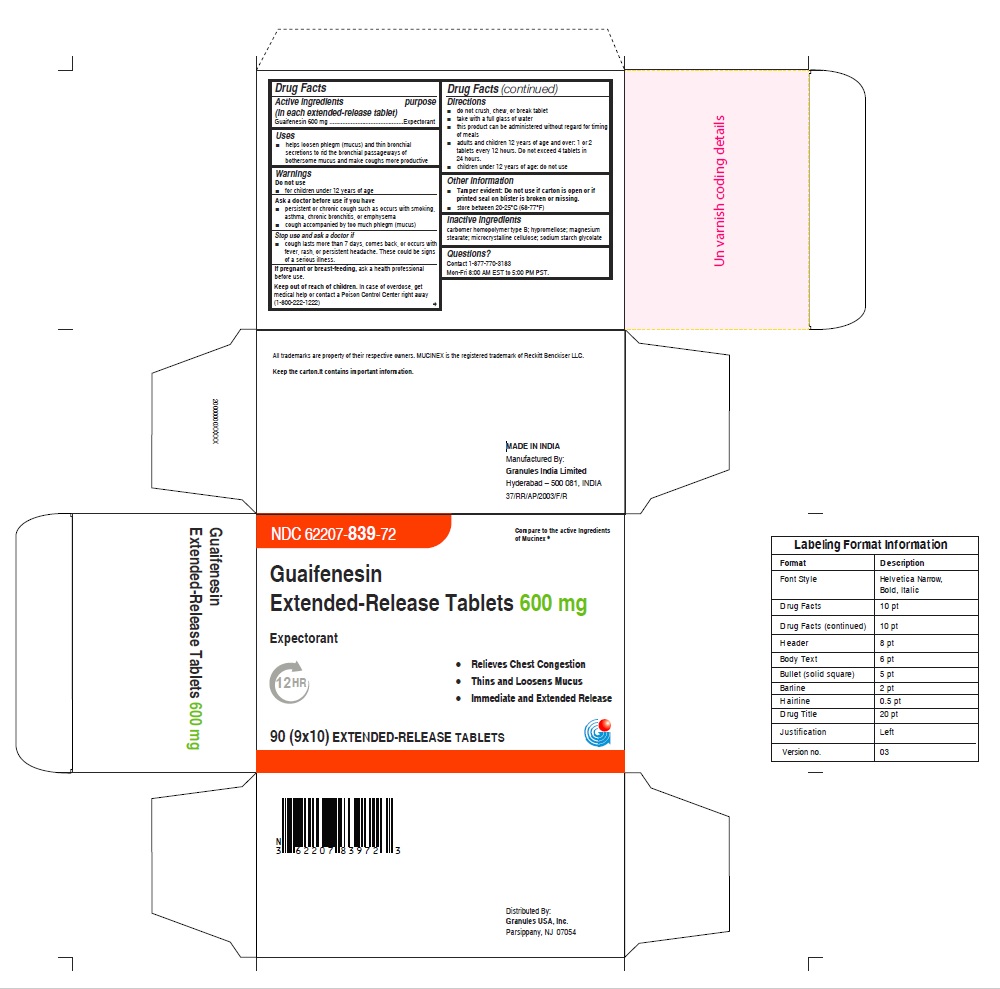
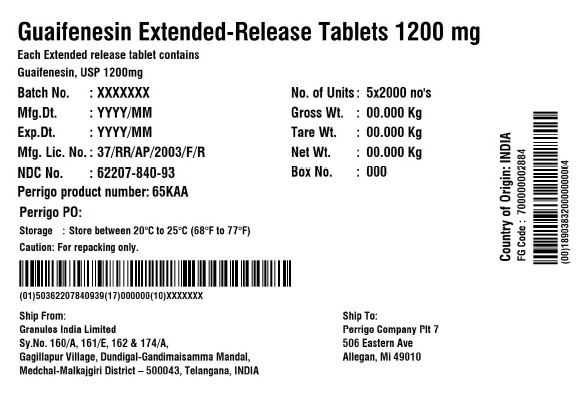
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUAIFENESIN
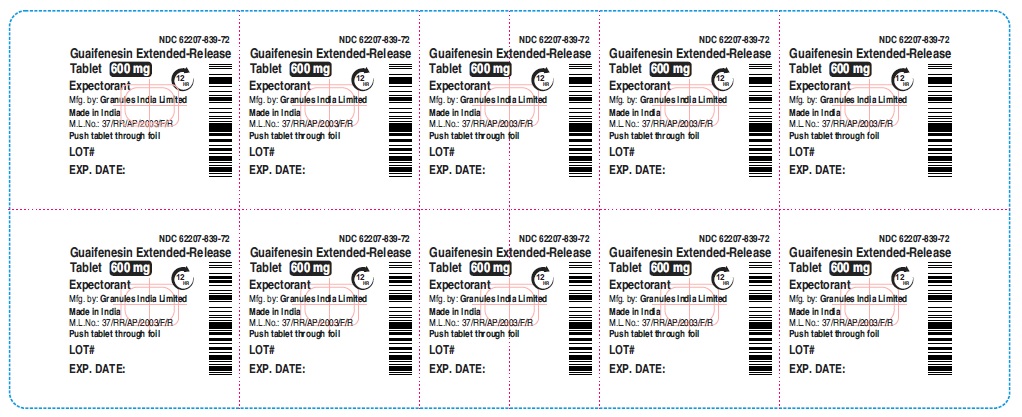
guaifenesin tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62207-839 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 600 mg Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER TYPE B (ALLYL SUCROSE CROSSLINKED) (UNII: Z135WT9208) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color white Score no score Shape OVAL Size 16mm Flavor Imprint Code G;600 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62207-839-72 9 in 1 CARTON 12/18/2020 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA213420 12/18/2020 GUAIFENESIN
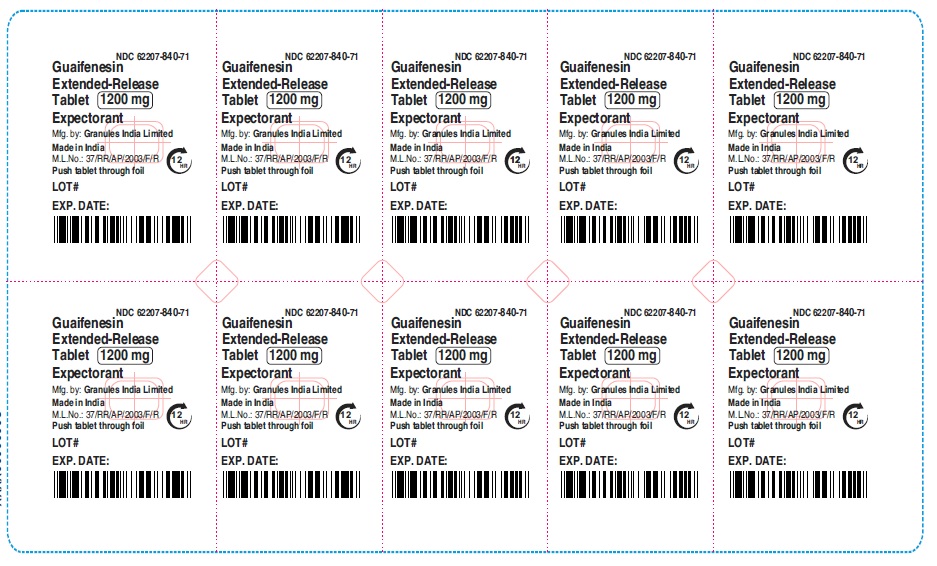
guaifenesin tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62207-840 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 1200 mg Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER TYPE B (ALLYL SUCROSE CROSSLINKED) (UNII: Z135WT9208) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color white Score no score Shape OVAL (Elliptical) Size 22mm Flavor Imprint Code G;1200 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62207-840-71 7 in 1 CARTON 12/18/2020 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:62207-840-99 4200 in 1 BAG; Type 0: Not a Combination Product 12/18/2020 3 NDC:62207-840-93 10000 in 1 BOX; Type 0: Not a Combination Product 10/31/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA213420 12/18/2020 Labeler - Granules India Ltd (915000087) Establishment Name Address ID/FEI Business Operations Granules India Ltd 918609236 analysis(62207-839, 62207-840) , label(62207-839, 62207-840) , manufacture(62207-839, 62207-840) , pack(62207-839, 62207-840)