Label: PREMIER VALUE EXTRA STRENGTH ORIGINAL FLAVOR- aluminum hydroxide and magnesium carbonate tablet, chewable
- NDC Code(s): 68016-105-10
- Packager: PHARMACY VALUE ALLIANCE, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 3, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
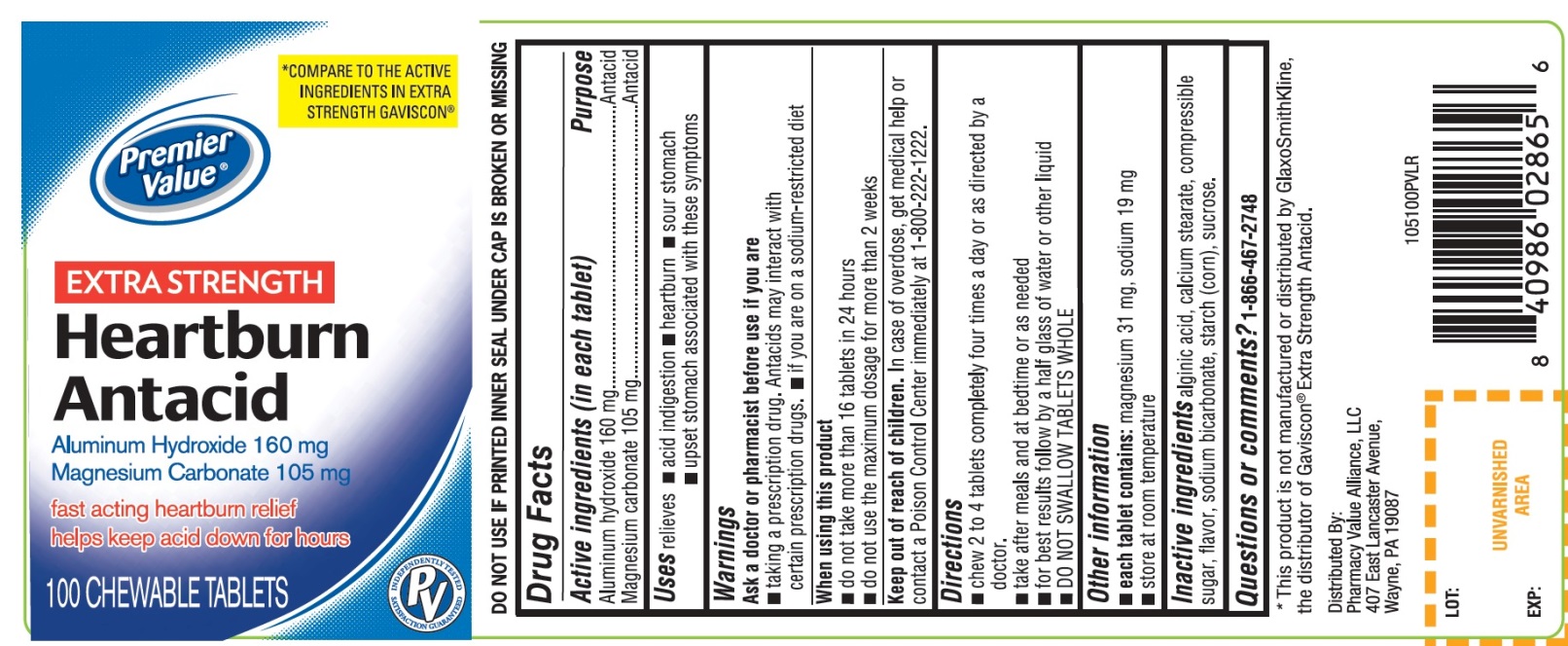
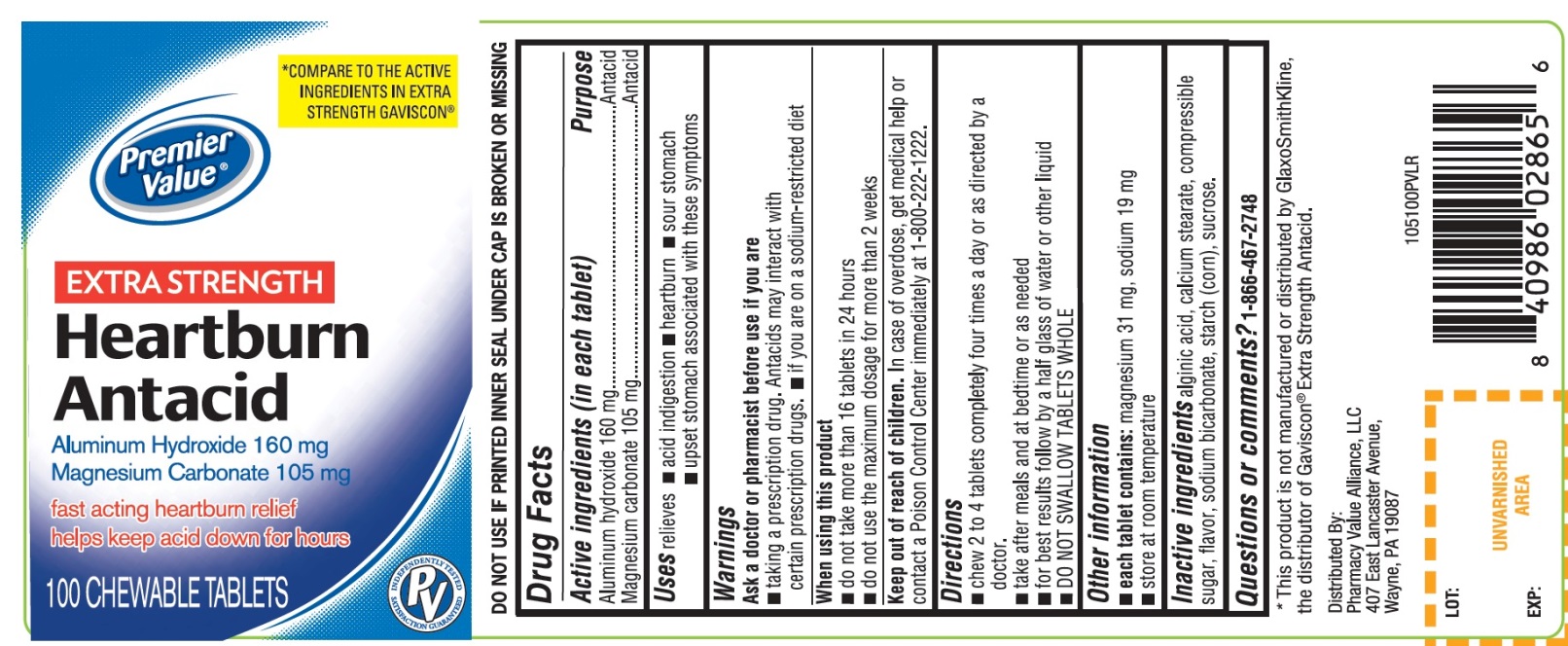
- Active ingredients (in each tablet)
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
-
Principal Display Panel
Premier Value®
NDC 68016-105-10
Heartburn Antacid
COMPARE TO THE ACTIVE INGREDIENTS IN EXTRA STRENGTH GAVISCON®
EXTRA STRENGTH
Aluminum Hydroxide, 160 mg
Magnesium Carbonate, 105 mg
- Fast-Acting Heartburn Relief
- Helps Keep Acid Down for Hours
100 CHEWABLE TABLETS
Distributed By:
Pharmacy Value Alliance, LLC
407 East Lancaster Avenue,
Wayne, PA 19087
DO NOT USE IF PRINTED INNER SEAL UNDER CAP IS BROKEN OR MISSING
If for any reason you are not satisfied with this product, please return it to the store where purchased for a full refund.
*This product is not manufactured or distributed by GlaxoSmithKline, the distributor of Gaviscon® Extra Strength Antacid.
-
INGREDIENTS AND APPEARANCE
PREMIER VALUE EXTRA STRENGTH ORIGINAL FLAVOR
aluminum hydroxide and magnesium carbonate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68016-105 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) (ALUMINUM HYDROXIDE - UNII:5QB0T2IUN0) ALUMINUM HYDROXIDE 160 mg MAGNESIUM CARBONATE (UNII: 0E53J927NA) (CARBONATE ION - UNII:7UJQ5OPE7D) MAGNESIUM CARBONATE 105 mg Inactive Ingredients Ingredient Name Strength ALGINIC ACID (UNII: 8C3Z4148WZ) CALCIUM STEARATE (UNII: 776XM7047L) CORN SYRUP (UNII: 9G5L16BK6N) SODIUM BICARBONATE (UNII: 8MDF5V39QO) STARCH, CORN (UNII: O8232NY3SJ) SUCROSE (UNII: C151H8M554) Product Characteristics Color WHITE Score no score Shape ROUND Size 17mm Flavor BUTTERSCOTCH (Original) Imprint Code RP105 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68016-105-10 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/20/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 03/20/2019 Labeler - PHARMACY VALUE ALLIANCE, LLC (101668460)