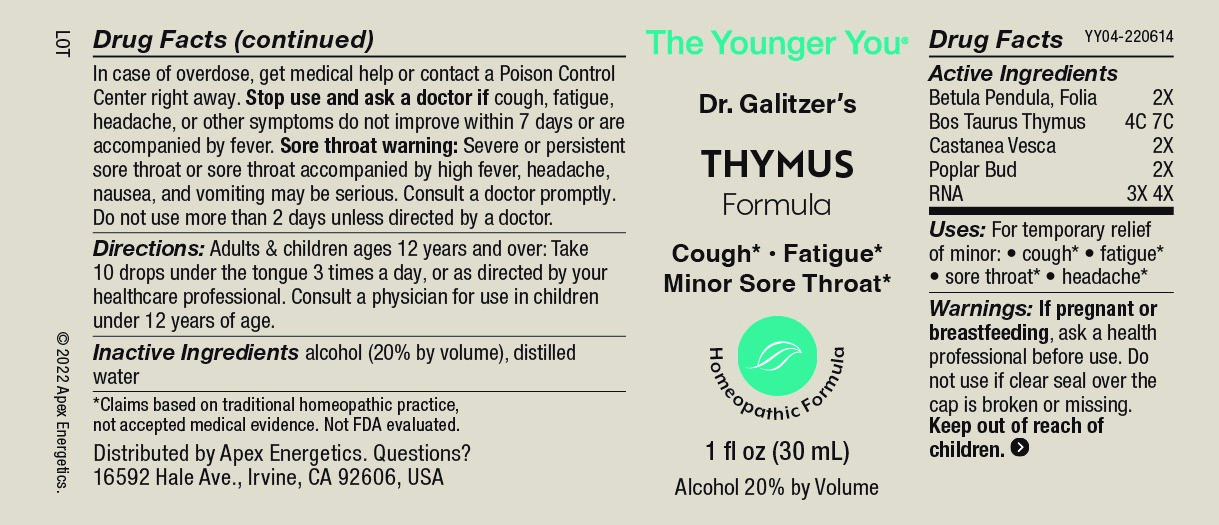
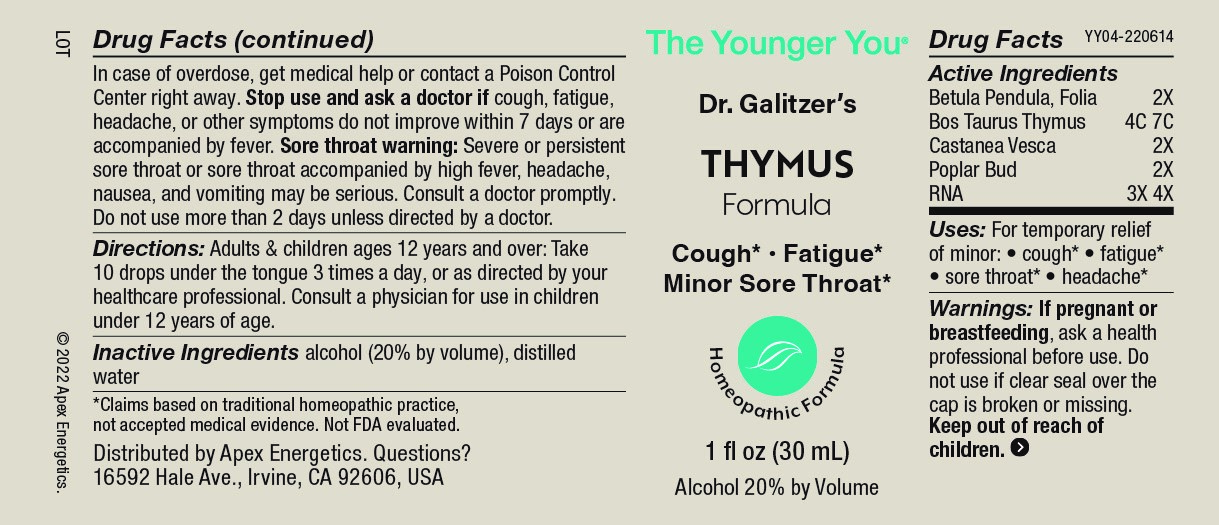
Label: THYMUS FORMULA- betula pendula folia bos taurus thymus, castanea vesca, poplar bud, rna solution/ drops
- NDC Code(s): 63479-2504-1
- Packager: Apex Energetics Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
-
Warnings:
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Stop use and ask a doctor if cough, fatigue, headache, or other symptoms do not improve within 7 days or are accompanied by fever.
Sore throat warning: Severe or persistent sore throat or sore throat accompanied by high fever, headache, nausea, and vomiting may be serious. Consult a doctor promptly. Do not use more than 2 days unless directed by a doctor.
- Directions:
- Inactive Ingredients
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
THYMUS FORMULA
betula pendula folia bos taurus thymus, castanea vesca, poplar bud, rna solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63479-2504 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POPULUS BALSAMIFERA LEAF BUD (UNII: 9CQ6C00G3C) (POPULUS BALSAMIFERA LEAF BUD - UNII:9CQ6C00G3C) POPULUS BALSAMIFERA LEAF BUD 2 [hp_X] in 1 mL SACCHAROMYCES CEREVISIAE RNA (UNII: J17GBZ5VGX) (SACCHAROMYCES CEREVISIAE RNA - UNII:J17GBZ5VGX) SACCHAROMYCES CEREVISIAE RNA 4 [hp_X] in 1 mL BETULA PENDULA LEAF (UNII: 5HW39H9KDH) (BETULA PENDULA LEAF - UNII:5HW39H9KDH) BETULA PENDULA LEAF 2 [hp_X] in 1 mL BOS TAURUS THYMUS (UNII: 8XEJ88V2T8) (BOS TAURUS THYMUS - UNII:8XEJ88V2T8) BOS TAURUS THYMUS 7 [hp_C] in 1 mL CASTANEA SATIVA LEAF (UNII: IV3S2HH53G) (CASTANEA SATIVA LEAF - UNII:IV3S2HH53G) CASTANEA SATIVA LEAF 2 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63479-2504-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 07/25/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 07/25/2022 Labeler - Apex Energetics Inc. (195816384) Registrant - Apex Energetics Inc. (195816384)