Label: AMIGLYDE-V- amikacin sulfate injection
- NDC Code(s): 54771-2332-1
- Packager: Zoetis Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated March 19, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Caution
-
DESCRIPTION
Amikacin sulfate is a semi-synthetic aminoglycoside
antibiotic derived from kanamycin. It is C22H43N5O13•2H2SO4, D-streptamine, 0-3-amino-3-
deoxy-α-D-glucopyranosyl-(1→6)-0-[6-amino-6- deoxy-α-D-glucopyranosyl-(1→4)]-N1-(4-amino-2-
hydroxy-1-oxobutyl)-2-deoxy-, (S)-, sulfate (1:2) (salt).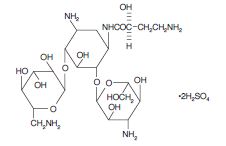
The dosage form supplied is a sterile, colorless solution.
The solution contains, in addition to amikacin sulfate, USP, 2.5% sodium citrate, USP with pH
adjusted to 4.5 with sulfuric acid and 0.66% sodium bisulfite added. The multi-dose 12 gram–48 mL
vial contains 0.01% benzethonium chloride, USP as a preservative.
-
ACTION
Antibacterial Activity
The effectiveness of AMIGLYDE-V (amikacin sulfate injection) in infections caused by Escherichia
coli, Pseudomonas sp and Klebsiella sp has been demonstrated clinically in the horse. In addition,
the following microorganisms have been shown to be susceptible to amikacin in vitro1, although the
clinical significance of this action has not been demonstrated in animals:
• Enterobacter sp
• Proteus mirabilis
• Proteus sp (indole positive)
• Serratia marcescens
• Salmonella sp
• Shigella sp
• Providencia sp
• Citrobacter freundii
• Listeria monocytogenes
ureus (both penicillin-
resistant and penicillin-sensitive)
The aminoglycoside antibiotics in general have limited
activity against gram-positive pathogens, although Staphylococcus aureus and Listeria monocytogenes
are susceptible to amikacin as noted above.Amikacin has been shown to be effective against
many aminoglycoside-resistant strains due to its
ability to resist degradation by aminoglycoside
inactivating enzymes known to affect gentamicin,
tobramycin and kanamycin2.
-
CLINICAL PHARMACOLOGY
Endometrial Tissue Concentrations
Comparisons of amikacin activity in endometrial biopsy tissue following intrauterine infusion with
that following intramuscular injection of AMIGLYDE-V in mares demonstrate superior endometrial
tissue concentrations when the drug is administered by
the intrauterine route.
Intrauterine infusion of 2 grams AMIGLYDE-V daily for three consecutive days in mares results in
peak concentrations typically exceeding 40 mcg/g of endometrial biopsy tissue within one hour after
infusion. Twenty-four hours after each treatment amikacin activity is still detectable at
concentrations averaging 2 to 4 mcg/g. However, the drug is not appreciably absorbed systemically
following intrauterine infusion. Endometrial tissue concentrations following intramuscular
injection are roughly parallel, but are typically somewhat lower
than corresponding serum concentrations of amikacin.
-
SAFETY
AMIGLYDE-V is non-irritating to equine endometrial
tissue when infused into the uterus as directed (see ADMINISTRATION AND DOSAGE). In laboratory
animals as well as equine studies, the drug was generally found not to be irritating when injected
intravenously, subcutaneously or intramuscularly.
Although amikacin, like other aminoglycosides, is potentially nephrotoxic, ototoxic and neurotoxic,
parenteral (intravenous) administration of AMIGLYDE-V (amikacin sulfate injection) twice daily at
dosages of up to 10 mg/lb for 15 consecutive days in horses resulted in no clinical, laboratory or
histopathologic evidence of toxicity.
Intrauterine infusion of 2 grams of AMIGLYDE-V
8 hours prior to breeding by natural service did not impair fertility in mares. Therefore, mares
should not be bred for at least 8 hours following uterine infusion.
-
INDICATIONS
AMIGLYDE-V is indicated for the treatment of uterine
infections (endometritis, metritis and pyometra) in mares, when caused by susceptible organisms
including Escherichia coli, Pseudomonas sp and Klebsiella sp. The use of AMIGLYDE-V in eliminating
infections caused by the above organisms has been shown clinically to improve fertility in infected
mares.
While nearly all strains of Escherichia coli,
Pseudomonas sp and Klebsiella sp, including those that are resistant to gentamicin, kanamycin or other
aminoglycosides, are susceptible to amikacin at levels achieved following treatment, it is
recommended that the invading organism be cultured and its susceptibility demonstrated as a guide
to therapy. Amikacin susceptibility discs, 30 mcg, should be used for determining in vitro susceptibility.
- ADMINISTRATION AND DOSAGE
- CONTRAINDICATIONS
- PRECAUTIONS
- ADVERSE REACTIONS
- WARNINGS
- HOW SUPPLIED
- REFERENCES
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - Carton Label
-
INGREDIENTS AND APPEARANCE
AMIGLYDE-V
amikacin sulfate injectionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:54771-2332 Route of Administration INTRAUTERINE Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMIKACIN SULFATE (UNII: N6M33094FD) (AMIKACIN - UNII:84319SGC3C) AMIKACIN 250 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-2332-1 1 in 1 CARTON 1 48 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA127892 02/01/2017 Labeler - Zoetis Inc. (828851555)