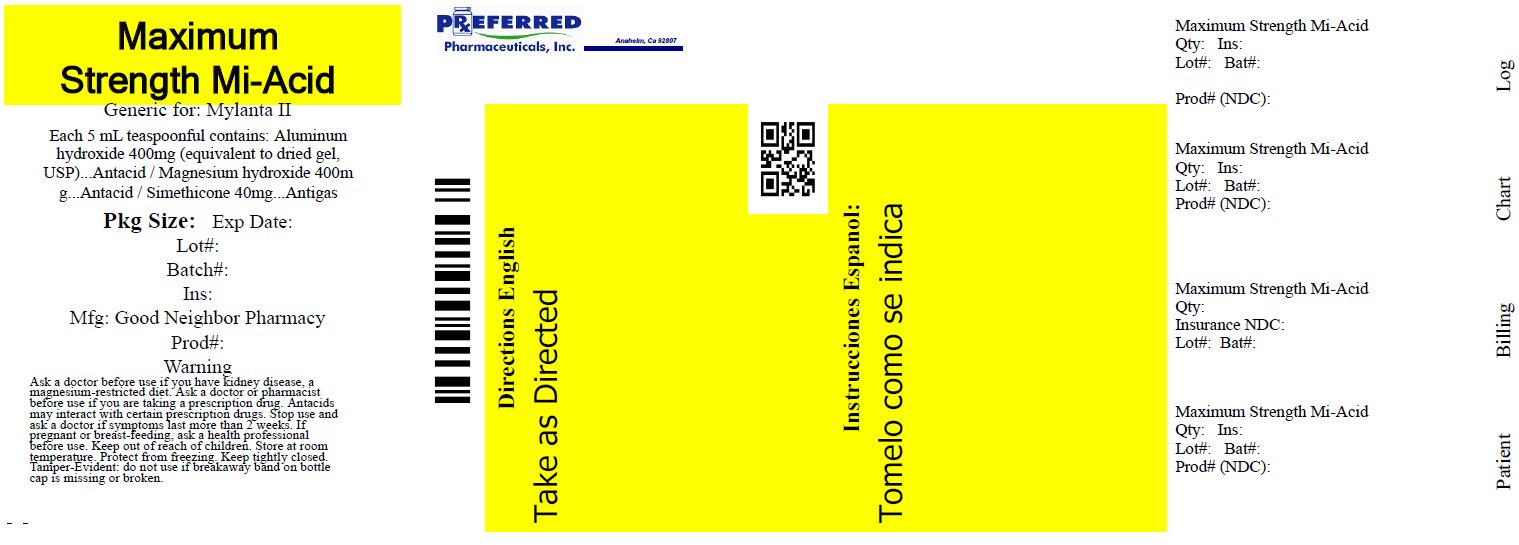
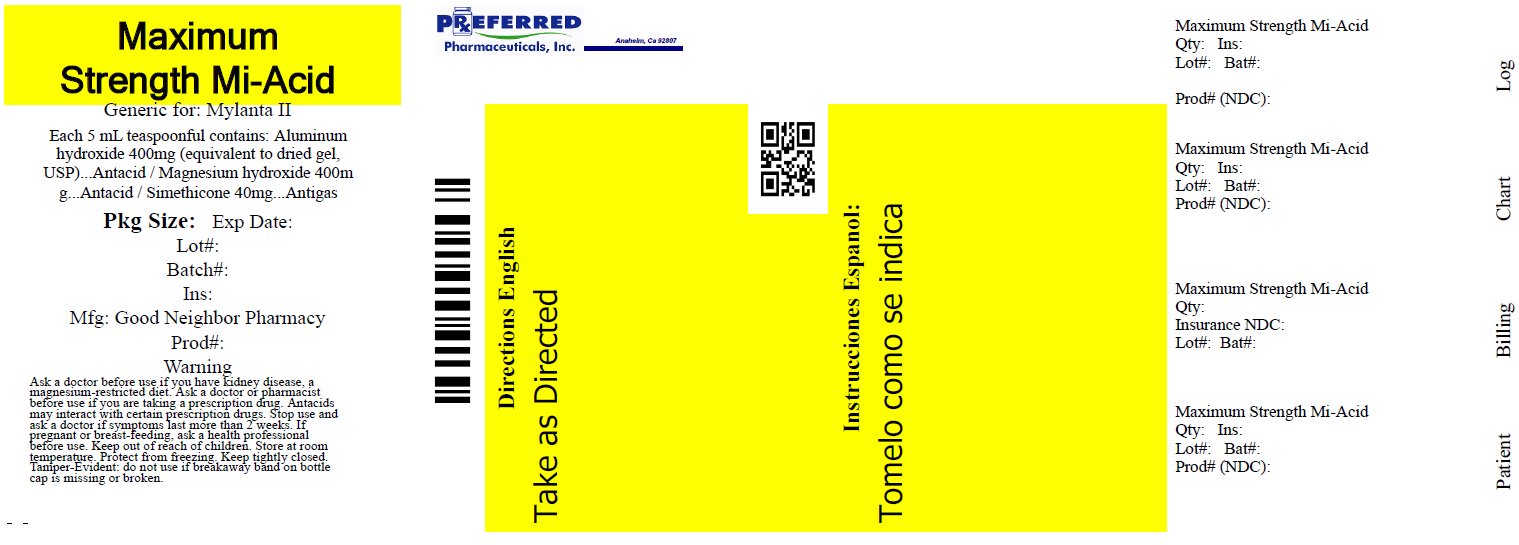
Label: ANTACID ANTIGAS NTA- aluminum hydroxide, magnesium hydroxide, simethicone liquid
- NDC Code(s): 68788-7641-3
- Packager: Preferred Pharmaceuticals, Inc.
- This is a repackaged label.
- Source NDC Code(s): 46122-432
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (in each 5 mL, 1 teaspoon)
- Purposes
- Uses
-
Warnings
Ask a doctor before use if you have
• kidney disease
• a magnesium-restricted diet
Ask a doctor or pharmacist before use if you are presently taking a prescription drug.
Antacids may interact with certain prescription drugs.
Stop use and ask a doctor if symptoms last more than 2 weeks
If pregnant or breast-feeding, ask a health professional before use.
- Directions
- Other information
- Inactive ingredients
-
package label
Relabeled by Preferred Pharmaceuticals, Inc.
Compare To Maalox® Maximum Strength active ingredients*
NDC 68788-7641-03
MAXIMUM STRENGTH
Antacid
Anti-GasAlumina, Magnesia, and Simethicone Oral Suspension
Fast Relief of:
• Acid Indigestion
• Heartburn
• Sour Stomach
• Pressure and Bloating
Cherry Creme Flavor12 FL OZ (355 mL)
-
INGREDIENTS AND APPEARANCE
ANTACID ANTIGAS NTA
aluminum hydroxide, magnesium hydroxide, simethicone liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68788-7641(NDC:46122-432) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) (ALUMINUM HYDROXIDE - UNII:5QB0T2IUN0) ALUMINUM HYDROXIDE 400 mg in 5 mL MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) (MAGNESIUM CATION - UNII:T6V3LHY838, HYDROXIDE ION - UNII:9159UV381P) MAGNESIUM HYDROXIDE 400 mg in 5 mL DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 40 mg in 5 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) BUTYLPARABEN (UNII: 3QPI1U3FV8) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SORBITOL (UNII: 506T60A25R) Product Characteristics Color Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68788-7641-3 355 mL in 1 BOTTLE; Type 0: Not a Combination Product 02/28/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 02/28/2020 Labeler - Preferred Pharmaceuticals, Inc. (791119022) Registrant - Preferred Pharmaceuticals, Inc. (791119022) Establishment Name Address ID/FEI Business Operations Preferred Pharmaceuticals, Inc. 791119022 RELABEL(68788-7641)