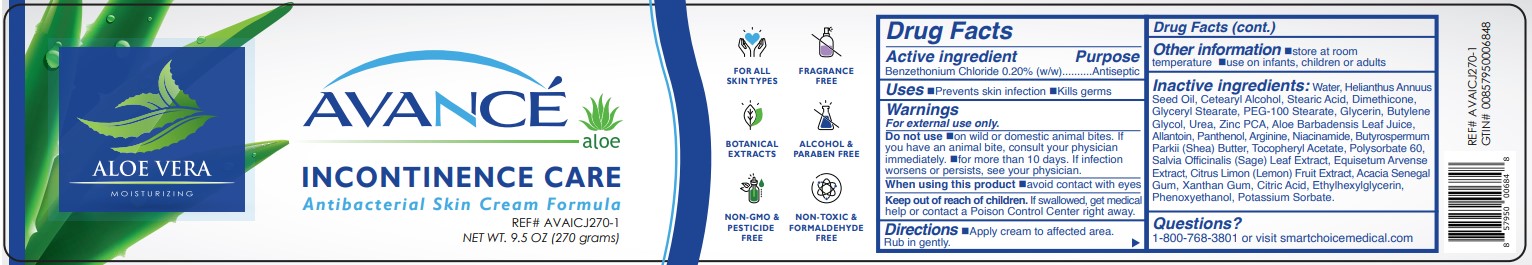
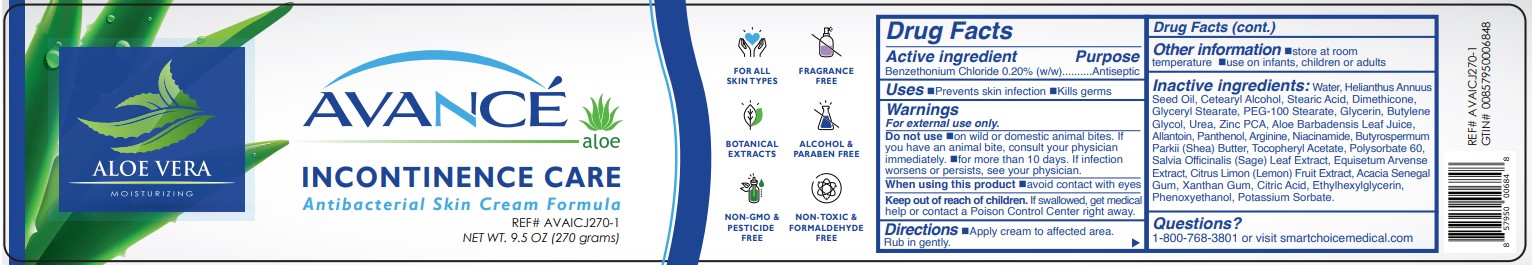
Label: AVANCE INCONTINENCE CARE- benzethonium chloride cream
- NDC Code(s): 70936-111-27, 70936-111-95
- Packager: SMART CHOICE MEDICAL INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT:
- PURPOSE
- USES
- WARNINGS:
- KEEP OUT OF REACH OF CHILDREN
- DIRECTIONS
- OTHER INFORMATION
-
INACTIVE INGREDIENTS
Water, Helianthus Annuus Seed Oil, Cetearyl Alcohol, Stearic Acid, Dimethicone, Glyceryl Stearate, PEG-100 Stearate, Glycerin, Butylene Glycol, Urea, Zinc PCA, Aloe Barbadensis Leaf Juice, Allantoin, Panthenol, Arginine, Niacinamide, Butyrospermum
Parkii (Shea) Butter, Tocopheryl Acetate, Polysorbate 60, Salvia Officinalis (Sage) Leaf Extract, Equisetum Arvense Extract, Citrus Limon (Lemon) Fruit Extract, Acacia Senegal Gum, Xanthan Gum, Citric Acid, Ethylhexylglycerin, Phenoxyethanol, Potassium Sorbate. - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AVANCE INCONTINENCE CARE
benzethonium chloride creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70936-111 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZETHONIUM CHLORIDE (UNII: PH41D05744) (BENZETHONIUM - UNII:1VU15B70BP) BENZETHONIUM CHLORIDE 0.2 g in 100 g Inactive Ingredients Ingredient Name Strength PANTHENOL (UNII: WV9CM0O67Z) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) STEARIC ACID (UNII: 4ELV7Z65AP) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) XANTHAN GUM (UNII: TTV12P4NEE) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) SHEA BUTTER (UNII: K49155WL9Y) GLYCERIN (UNII: PDC6A3C0OX) PEG-100 STEARATE (UNII: YD01N1999R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) UREA (UNII: 8W8T17847W) POLYSORBATE 60 (UNII: CAL22UVI4M) ZINC PIDOLATE (UNII: C32PQ86DH4) ALLANTOIN (UNII: 344S277G0Z) ARGININE (UNII: 94ZLA3W45F) SUNFLOWER OIL (UNII: 3W1JG795YI) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) NIACINAMIDE (UNII: 25X51I8RD4) SAGE (UNII: 065C5D077J) LEMON JUICE (UNII: AGN709ANTJ) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) ACACIA (UNII: 5C5403N26O) EQUISETUM ARVENSE WHOLE (UNII: 73DM367W4P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70936-111-95 270 g in 1 JAR; Type 0: Not a Combination Product 06/29/2022 2 NDC:70936-111-27 270 g in 1 TUBE; Type 0: Not a Combination Product 06/29/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 06/29/2022 Labeler - SMART CHOICE MEDICAL INC. (046383276)