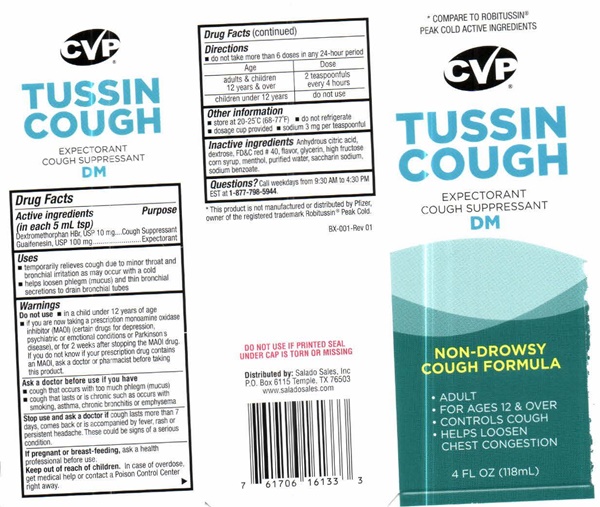
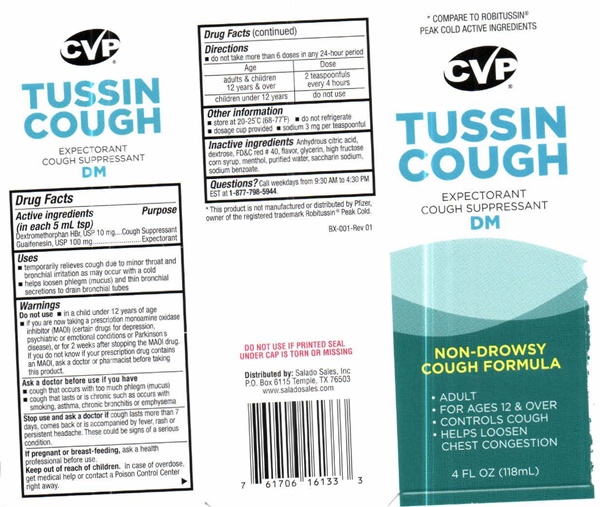
Label: TUSSIN COUGH EXPECTORANT COUGH SUPRESSANT DM- dextromethorphan hydrobromide, guaifenesin liquid
- NDC Code(s): 57243-503-24
- Packager: Salado Sales, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug FactsActive ingredients ( in each 5 mL tsp)
- Purpose
- Keep out of reach of children.
- Uses
-
Warnings
Do not use
- in a child under 12 years of age
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product
- Ask A Doctor Before You Use if You Have
- Stop Use And Ask A Doctor If
- If Pregnant Or Breast-Feeding
- Directions
- Other information
- Inactive ingredients
- Questions?
-
Tussin Cough DM product label
* COMPARE TO ROBITUSSIN® PEAK COLD ACTIVE INGREDIENTS
CVP® CONSUMER VALUE PRODUCTS
TUSSIN COUGH EXPECTORANT COUGH SUPPRESSANT DMNON-DROWSY COUGH FORMULA
- ADULT
- FOR AGES 12 AND OVER
- CONTROLS COUGH
- HELPS LOOSEN CHEST CONGESTION
4 FL OZ (118 Ml)
* This product is not manufactured or distributed by Pfizer, owner of the registered trademark Robitussin® Peak Cold
Box-001 Rev 01
DO NOT USE IF PRINTED SEAL UNDER CAP IS TORN OR MISSING
Distributed by: Salado Sales, Inc. P.O. Box 6115 Temple, TX 76503 www.saladosales.comLOT.
EXP.

res


-
INGREDIENTS AND APPEARANCE
TUSSIN COUGH EXPECTORANT COUGH SUPRESSANT DM
dextromethorphan hydrobromide, guaifenesin liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57243-503 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg in 5 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 100 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) DEXTROSE (UNII: IY9XDZ35W2) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) MENTHOL (UNII: L7T10EIP3A) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM BENZOATE (UNII: OJ245FE5EU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57243-503-24 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 01/01/2012 Labeler - Salado Sales, Inc. (009830555) Registrant - AptaPharma Inc. (790523323) Establishment Name Address ID/FEI Business Operations AptaPharma Inc. 790523323 manufacture(57243-503)