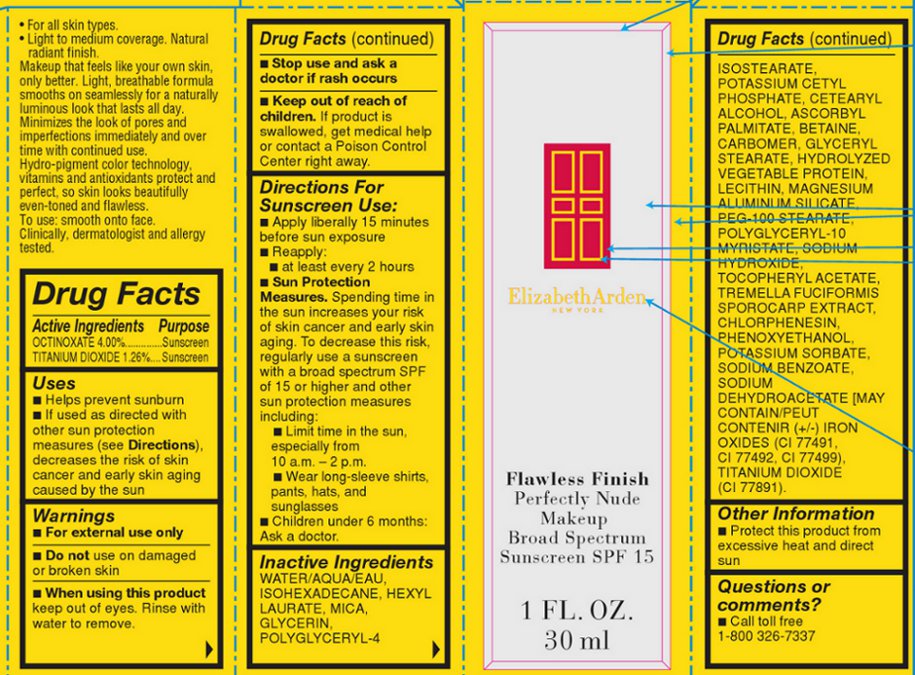
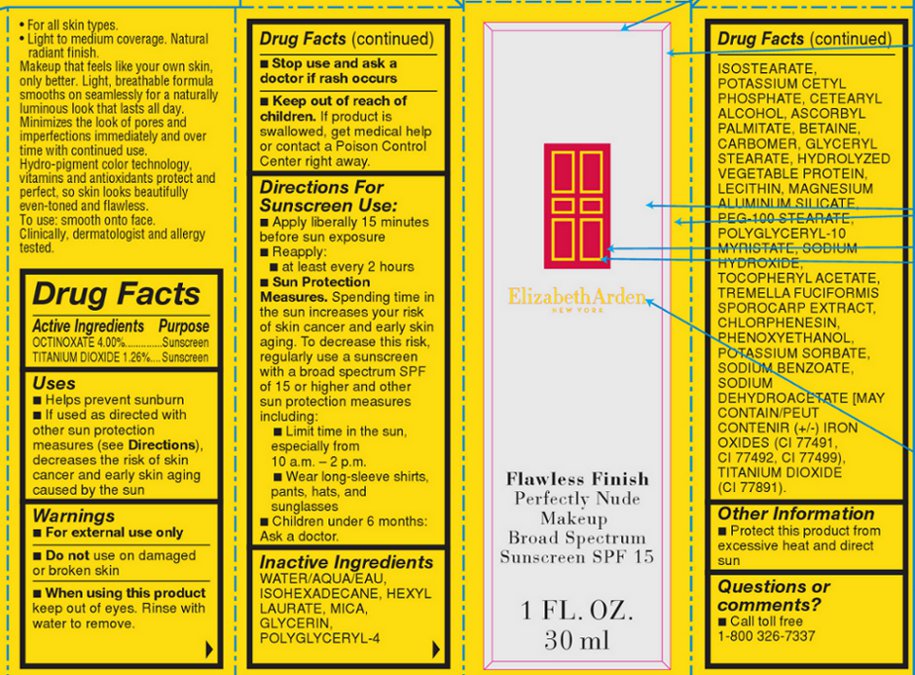
Label: FLAWLESS FINISH PERFECTLY NUDE MAKEUP BROAD SPECTRUM SUNSCREEN SPF 15 SHADE COCOA- octinoxate and titanium dioxide lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 67938-2027-1, 67938-2027-2 - Packager: Elizabeth Arden, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 16, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Makeup that feels like your own skin, only better. Light, breathable formula smooths on seamlessly for a naturally luminous look that lasts all day. Minimizes the look of pores and imperfections immediately and over time with continued use. Hydro-pigment color technology, vitamins and antioxidants protect and perfect, so skin looks beautifully even-toned and flawless.
- INDICATIONS & USAGE
- WARNINGS
- OTC - ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
WATER/AQUA/EAU, ISOHEXADECANE, HEXYL LAURATE, MICA, GLYCERIN, POLYGLYCERYL-4 ISOSTEARATE, POTASSIUM CETYL PHOSPHATE, CETEARYL ALCOHOL, ASCORBYL PALMITATE, BETAINE, CARBOMER, GLYCERYL STEARATE, HYDROLYZED VEGETABLE PROTEIN, LECITHIN, MAGNESIUM ALUMINUM SILICATE, PEG-100 STEARATE, POLYGLYCERYL-10 MYRISTATE, SODIUM HYDROXIDE, TOCOPHERYL ACETATE, TREMELLA FUCIFORMIS SPOROCARP EXTRACT, CHLORPHENESIN, PHENOXYETHANOL, POTASSIUM SORBATE, SODIUM BENZOATE, SODIUM DEHYDROACETATE [MAY CONTAIN/PEUT CONTENIR (+/-) IRON OXIDES (CI 77491, CI 77492, CI 77499), TITANIUM DIOXIDE (CI 77891).
- OTC - DO NOT USE
- OTC - KEEP OUT OF REACH OF CHILDREN
- OTC - PURPOSE
- OTC - STOP USE
- OTC - WHEN USING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FLAWLESS FINISH PERFECTLY NUDE MAKEUP BROAD SPECTRUM SUNSCREEN SPF 15 SHADE COCOA
octinoxate and titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67938-2027 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 60 mg in 1500 mg TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 18.9 mg in 1500 mg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISOHEXADECANE (UNII: 918X1OUF1E) HEXYL LAURATE (UNII: 4CG9F9W01Q) MICA (UNII: V8A1AW0880) GLYCERIN (UNII: PDC6A3C0OX) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) ASCORBYL PALMITATE (UNII: QN83US2B0N) BETAINE (UNII: 3SCV180C9W) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) PEG-100 STEARATE (UNII: YD01N1999R) SODIUM HYDROXIDE (UNII: 55X04QC32I) CHLORPHENESIN (UNII: I670DAL4SZ) PHENOXYETHANOL (UNII: HIE492ZZ3T) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) Product Characteristics Color BROWN (BROWN) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67938-2027-1 1 in 1 BOX 1 NDC:67938-2027-2 1500 mg in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 07/17/2013 Labeler - Elizabeth Arden, Inc (849222187) Establishment Name Address ID/FEI Business Operations Intercos 883457061 manufacture(67938-2027)