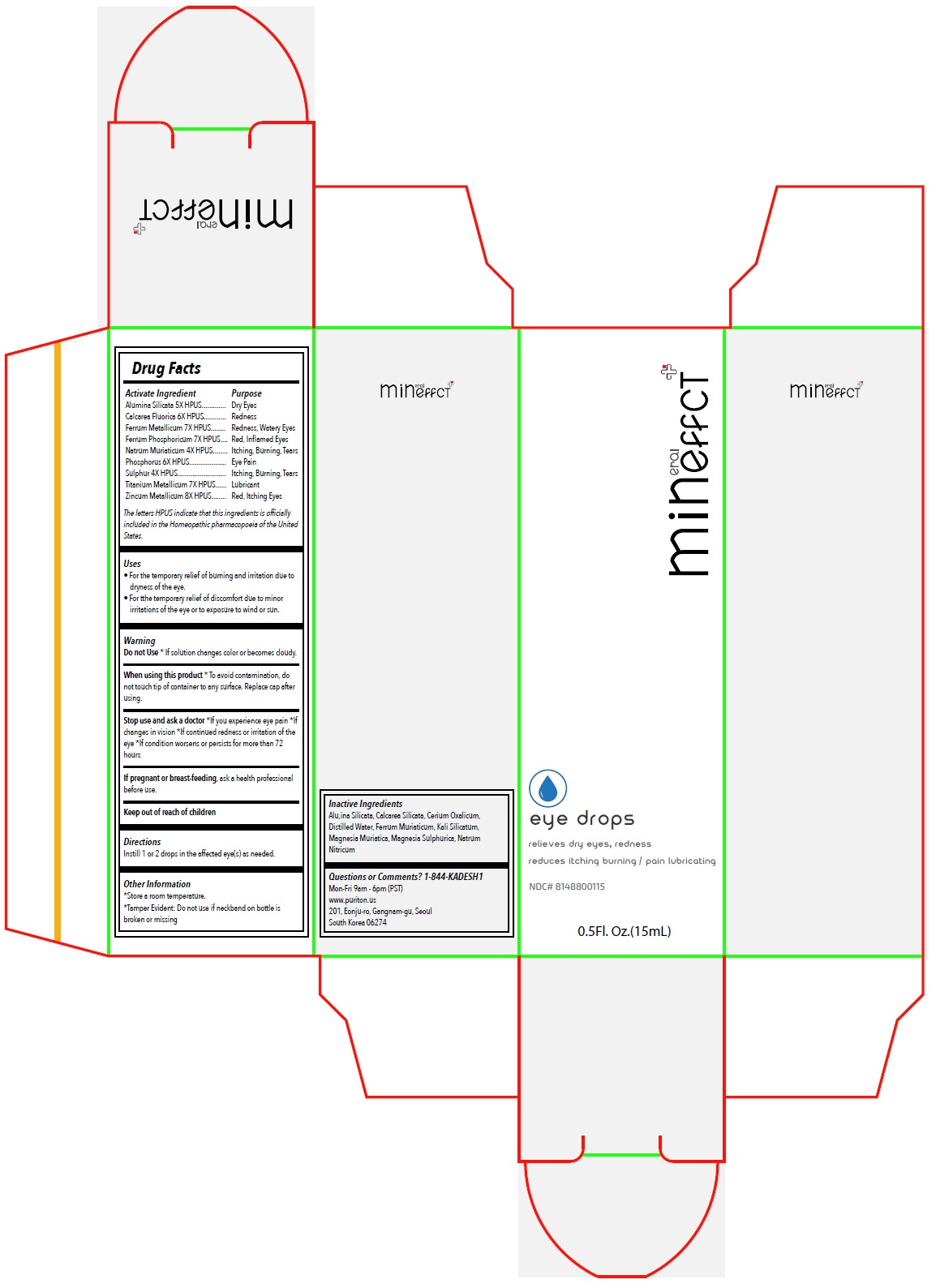
Label: MINEFFECT EYE DROPS- alumina silicata, calcarea fluorica, ferrum metallicum, ferrum phosphoricum, natrum muriaticum, phosphorus, sulphur, titanium metallicum, zincum metallicum solution/ drops
- NDC Code(s): 81488-001-15
- Packager: Kadesh Incoporation Co,Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated April 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
-
Activate Ingredient
Alumina Silicata 5X HPUS
Calcarea Fluorica 6X HPUS
Ferrum Metallicum 7X HPUS
Ferrum Phosphoricum 7X HPUS
Natrum Muriaticum 4X HPUS
Phosphorus 6X HPUS
Sulphur 4X HPUS
Titanium Metallicum 7X HPUS
Zincum Metallicum 8X HPUSThe letters HPUS indicate that this ingredients is officially included in the Homeopathic pharmacopoeia of the United States.
- Purpose
- Uses
-
Warning
Do not Use* If solution changes color or becomes cloudy.
When using this product* To avoid contamination, do not touch tip of container to any surface. Replace cap after using.
Stop use and ask a doctor*If you experience eye pain *If changes in vision *If continued redness or irritation of the eye *If condition worsens or persists for more than 72 hours
If pregnant or breast-feeding, ask a health professional before use.
- Directions
- Other Information
- Inactive Ingredients
- Questions or Comments? 1-844-KADESH1
- SPL UNCLASSIFIED SECTION
- Packaging
-
INGREDIENTS AND APPEARANCE
MINEFFECT EYE DROPS
alumina silicata, calcarea fluorica, ferrum metallicum, ferrum phosphoricum, natrum muriaticum, phosphorus, sulphur, titanium metallicum, zincum metallicum solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81488-001 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength KAOLIN (UNII: 24H4NWX5CO) (KAOLIN - UNII:24H4NWX5CO) KAOLIN 5 [hp_X] in 15 mL CALCIUM FLUORIDE (UNII: O3B55K4YKI) (FLUORIDE ION - UNII:Q80VPU408O) CALCIUM FLUORIDE 6 [hp_X] in 15 mL IRON (UNII: E1UOL152H7) (IRON - UNII:E1UOL152H7) IRON 7 [hp_X] in 15 mL FERROSOFERRIC PHOSPHATE (UNII: 91GQH8I5F7) (FERROSOFERRIC PHOSPHATE - UNII:91GQH8I5F7) FERROSOFERRIC PHOSPHATE 7 [hp_X] in 15 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 4 [hp_X] in 15 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 6 [hp_X] in 15 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 4 [hp_X] in 15 mL TITANIUM (UNII: D1JT611TNE) (TITANIUM - UNII:D1JT611TNE) TITANIUM 7 [hp_X] in 15 mL ZINC (UNII: J41CSQ7QDS) (ZINC - UNII:J41CSQ7QDS) ZINC 8 [hp_X] in 15 mL Inactive Ingredients Ingredient Name Strength CALCIUM SILICATE (UNII: S4255P4G5M) CEROUS OXALATE NONAHYDRATE (UNII: 0UV74P3R0J) WATER (UNII: 059QF0KO0R) FERRIC CHLORIDE HEXAHYDRATE (UNII: 0I2XIN602U) POTASSIUM SILICATE (UNII: J86L1GUL6K) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) MAGNESIUM SULFATE HEPTAHYDRATE (UNII: SK47B8698T) SODIUM NITRATE (UNII: 8M4L3H2ZVZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81488-001-15 1 in 1 BOX 02/01/2021 1 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/01/2021 Labeler - Kadesh Incoporation Co,Ltd (694615354) Establishment Name Address ID/FEI Business Operations Kadesh Incoporation Co,Ltd 694615354 manufacture(81488-001)