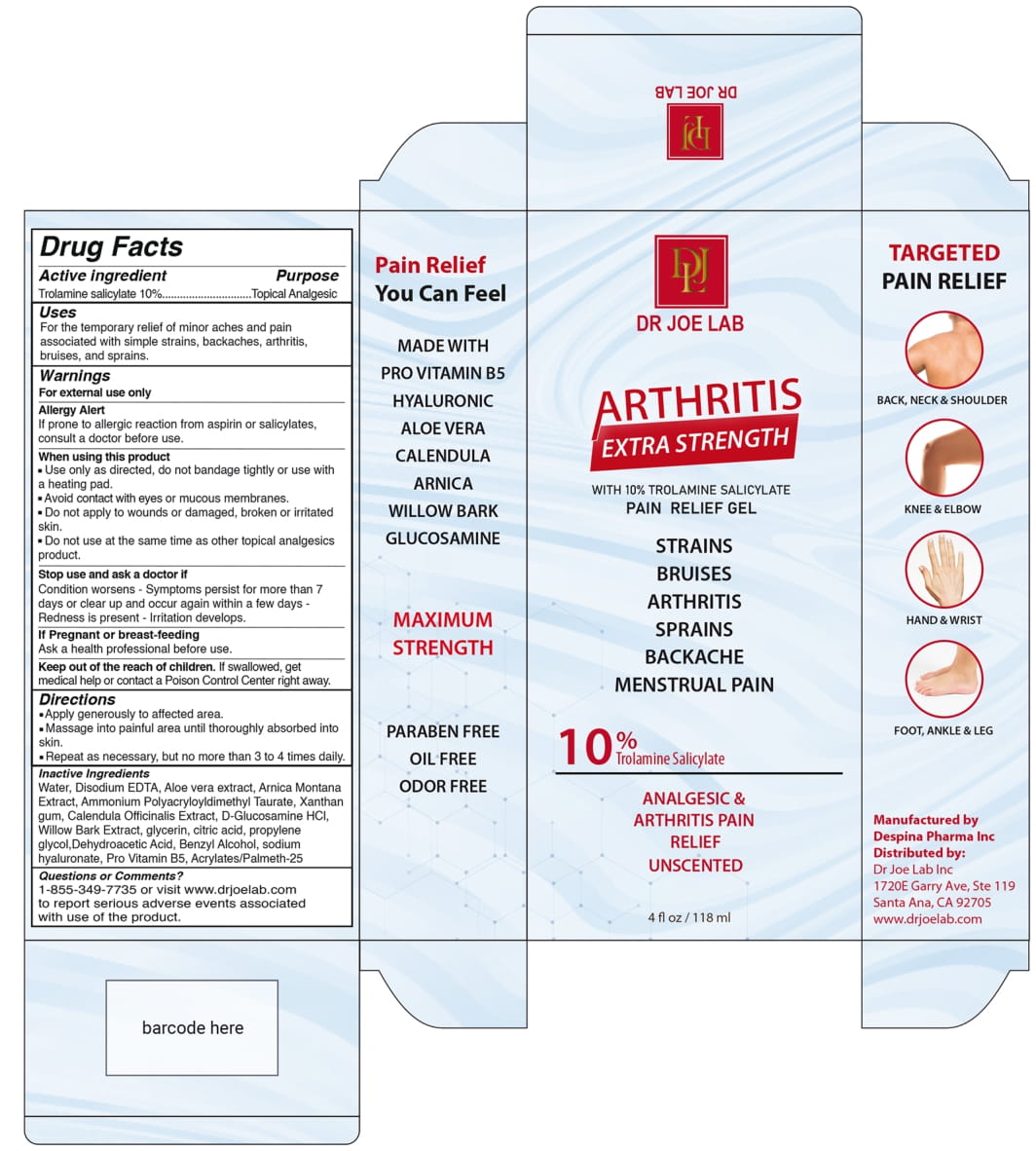
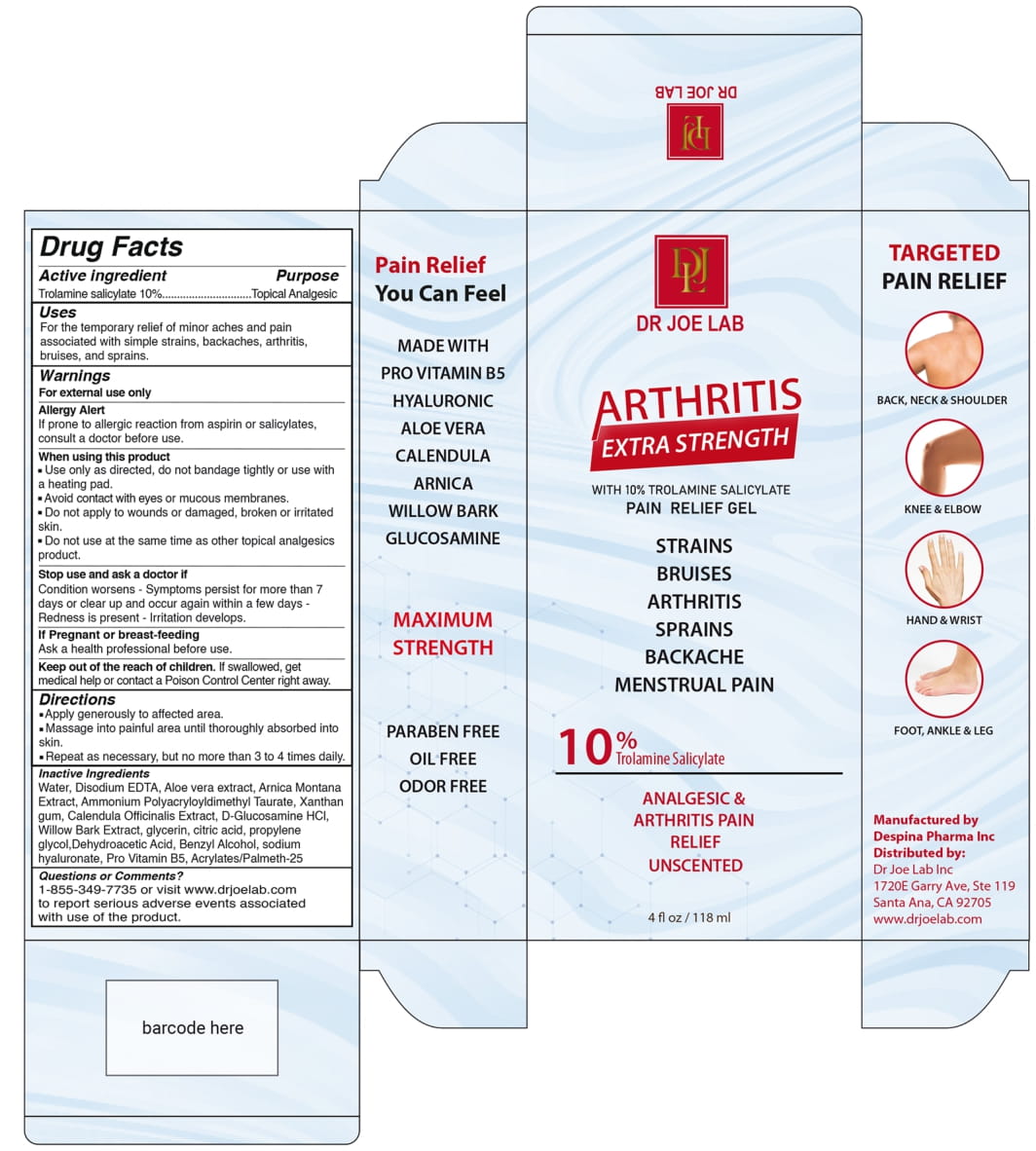
Label: DR JOE LAB ARTHRITIS EXTRA STRENGTH- trolamine salicylate gel
- NDC Code(s): 82330-401-01
- Packager: DESPINA PHARMA INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Warnings
- WHEN USING
- ASK DOCTOR
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Water, Disodium EDTA, Aloe vera extract, Arnica Montana Extract, Ammonium Polyacryloyldimethyl Taurate, Xanthan gum. Calendula Officinalis Extract , D-Glucosamin Hcl, Willow Bark Extract, Glycerine, Citric acid, propylene glycol, Dehydroacetic Acid, Benzyl Alcohol, Sodium hyaluronate, Pro Vitamin B5, Acrylates/Palmeth-25
- QUESTIONS
- INDICATIONS & USAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DR JOE LAB ARTHRITIS EXTRA STRENGTH
trolamine salicylate gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82330-401 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TROLAMINE SALICYLATE (UNII: H8O4040BHD) (SALICYLIC ACID - UNII:O414PZ4LPZ) TROLAMINE SALICYLATE 10 g in 100 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) EDETATE DISODIUM (UNII: 7FLD91C86K) ARNICA MONTANA (UNII: O80TY208ZW) AMMONIUM POLYACRYLOYLDIMETHYL TAURATE (55000 MPA.S) (UNII: F01RIY4371) XANTHAN GUM (UNII: TTV12P4NEE) CALENDULA OFFICINALIS WHOLE (UNII: PFR03EBU0H) GLUCOSAMINE HYDROCHLORIDE (UNII: 750W5330FY) WILLOW BARK (UNII: S883J9JDYX) GLYCERIN (UNII: PDC6A3C0OX) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) DEHYDROACETIC ACID (UNII: 2KAG279R6R) BENZYL ALCOHOL (UNII: LKG8494WBH) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DEXPANTHENOL (UNII: 1O6C93RI7Z) METHACRYLIC ACID AND ETHYL ACRYLATE COPOLYMER (UNII: NX76LV5T8J) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82330-401-01 118 mL in 1 TUBE; Type 0: Not a Combination Product 06/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M014 06/01/2022 Labeler - DESPINA PHARMA INC (112281681) Establishment Name Address ID/FEI Business Operations Despina Pharma Inc 112281681 manufacture(82330-401)