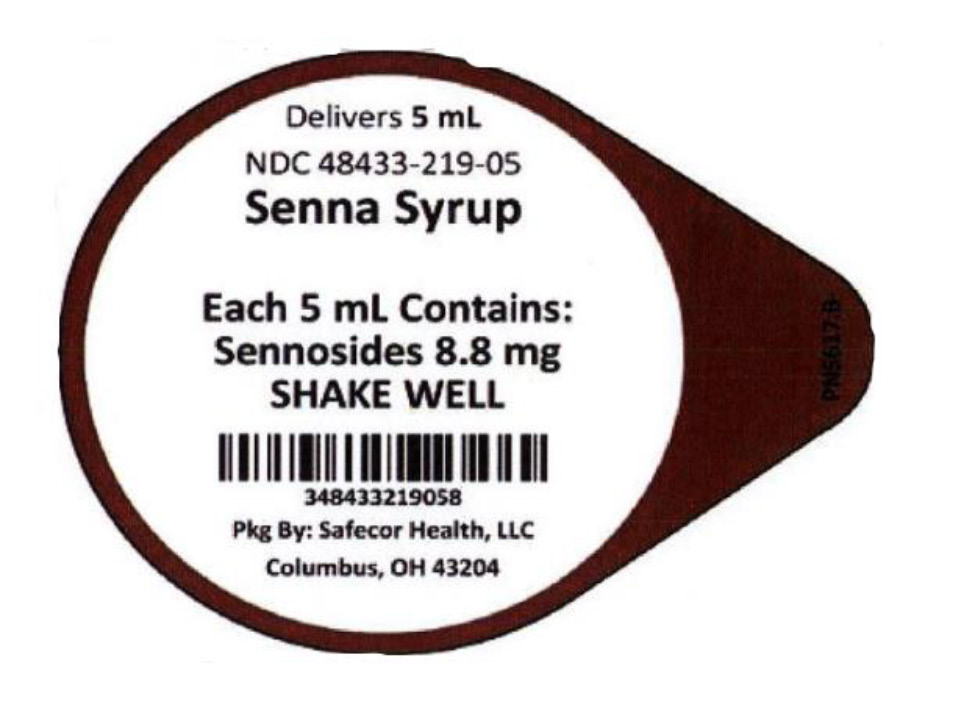
Label: SENNA SYRUP- sennosides liquid
- NDC Code(s): 48433-219-05, 48433-219-40
- Packager: Safecor Health, LLC
- This is a repackaged label.
- Source NDC Code(s): 54859-808
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- ASK DOCTOR
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
1 teaspoonful = 5 mL Directions: - Shake well before use
- Do not exceed recommended dose
Age Starting Dose Maximum Dosage Adults and children 12 years of age and over 2 to 3 teaspoonfuls (10 mL to 15 mL) once a day preferably at bedtime; increase as needed or as recommended by a doctor 3 teaspoonfuls (15 mL) in the morning and 3 teaspoonfuls (15 mL) at bedtime Children under 12 years of age Ask a doctor Ask a doctor - STORAGE AND HANDLING
- INACTIVE INGREDIENT
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL
———Principal Display Panel———
SAFECOR HEALTH
NDC: 48433-219-40
Senna Syrup Qty: 40
8.8 mg Sennosides per 5 mL Lot: 20A0020
Contains 40 (5 mL) Unit Dose Cups Exp: 210203
See monograph for complete drug information.
Store at room temperature 15°C to 25°C (59°F to 77°F);
excursions between 26°C to 30°C (78°F to 86°F) are allowed.
Protect from excessive heat.
This package design is not child resistant. For institutional use only.
Shake well before use.
GTIN: 00348433219409
SN: 200600019
Pkg By: Safecor Health, LLC Columbus, OH 43204 Exp: 210203
Questions or Comments: Call 1-800-447-1006 Lot: 20A0020
-
INGREDIENTS AND APPEARANCE
SENNA SYRUP
sennosides liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:48433-219(NDC:54859-808) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 8.8 mg in 5 mL Inactive Ingredients Ingredient Name Strength METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SUCROSE (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:48433-219-40 40 in 1 BOX 03/01/2020 1 NDC:48433-219-05 5 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 03/01/2020 Labeler - Safecor Health, LLC (828269675) Establishment Name Address ID/FEI Business Operations Safecor Health, LLC 828269675 repack(48433-219) Establishment Name Address ID/FEI Business Operations Llorens Pharmaceutical International Division, Inc. 037342305 manufacture(48433-219)