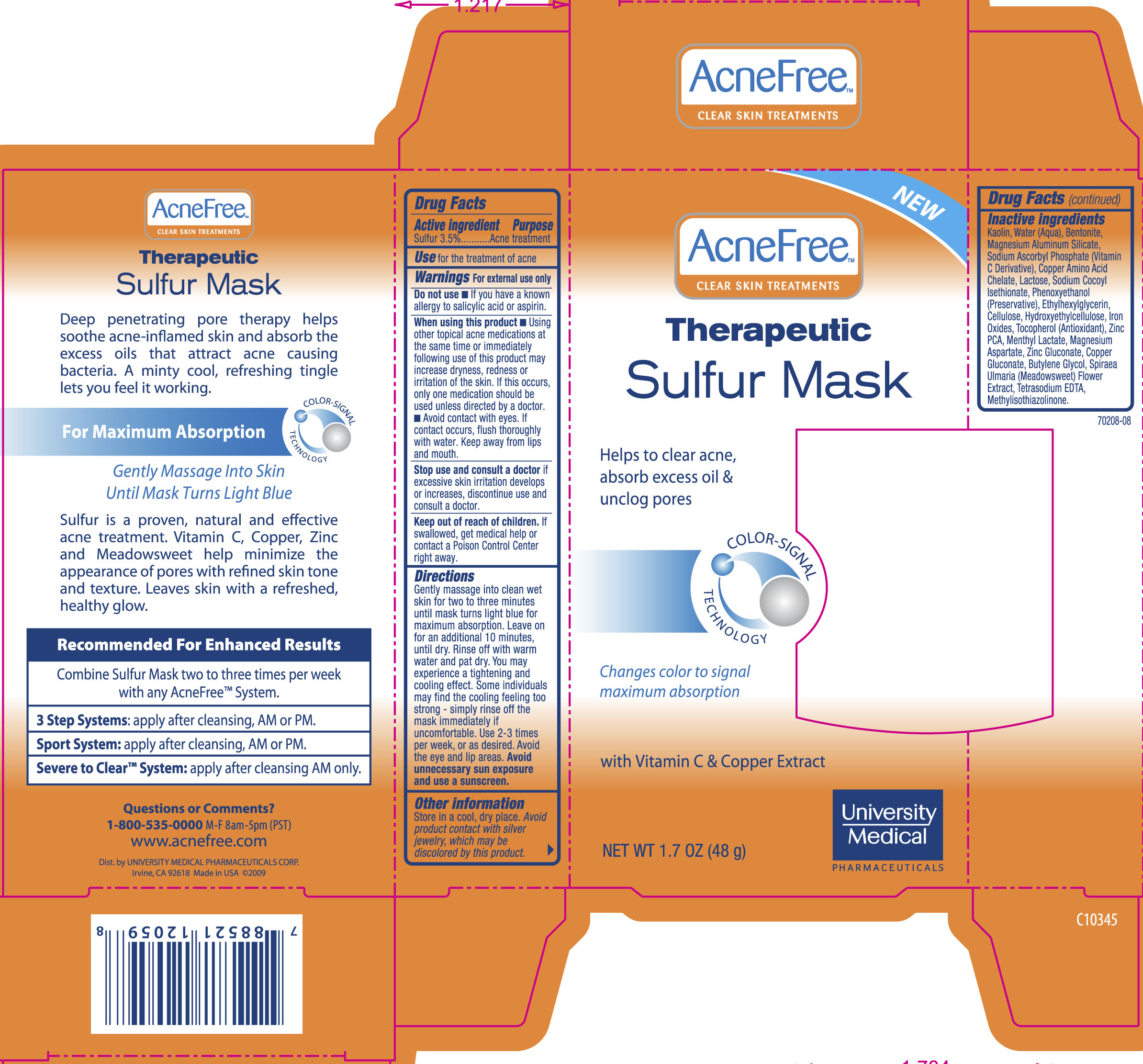
Label: THERAPEUTIC SULFUR MASK- sulfur cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 50544-004-02, 50544-004-91 - Packager: University Medical Pharmaceuticals Corp
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 12, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
-
WHEN USING
When using this product
- Using other topical acne medications at the same time or immediately following use of this product may increase dryness, redness or irritation of the skin. If this occurs, only one medication should be used unless directed by a doctor.
- Avoid contact with lips and mouth. Do not get into eyes. If contact occurs, flush thoroughly with water. Keep away from lips and mouth.
- Using other topical acne medications at the same time or immediately following use of this product may increase dryness, redness or irritation of the skin. If this occurs, only one medication should be used unless directed by a doctor.
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
Gently massage into clean wet skin for two to three minutes until mask turns light blue for maximum absorption. Leave on for an additional 10 minutes, until dry. Rinse off with warm water and pat dry. You may experience a tightening and cooling effect. Some individuals may find the cooling feeling too strong - simply rinse off the mask immediately if uncomfortable. Use 2-3 times per week, or as desired. Avoid eye and lip areas. Avoid unnecessary sun exposure and use a sunscreen. -
INACTIVE INGREDIENT
Inactive Ingredients
Acacia Senegal Gum, Bentonite, Butylene Glycol, Cellulose, Copper Gluconate, Ethylhexylglycerin, Hydroxypropyl Methylcellulose, Kaolin, Magnesium Aluminum Silicate, Magnesium Aspartate, Magnesium Stearate, Mannitol, Menthyl Lactate, Methylisothiazolinone, Phenoxyethanol, Soidum Ascobyl Phosphate, Sodium Cocoyl Isethionate, Spiraea Ulmaria Flower Extract, Tetrasodium EDTA, Titanium Dioxide, Ultramarines (CI 77007), Water/Aqua/Eau, Zinc PCA.
- PURPOSE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
THERAPEUTIC SULFUR MASK
sulfur creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50544-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sulfur (UNII: 70FD1KFU70) (Sulfur - UNII:70FD1KFU70) Sulfur 35 g in 1000 g Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) kaolin (UNII: 24H4NWX5CO) bentonite (UNII: A3N5ZCN45C) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) titanium dioxide (UNII: 15FIX9V2JP) ACACIA (UNII: 5C5403N26O) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) SODIUM COCOYL ISETHIONATE (UNII: 518XTE8493) ethylhexylglycerin (UNII: 147D247K3P) HYDROXYPROPYL CELLULOSE (UNII: RFW2ET671P) menthyl lactate (UNII: 2BF9E65L7I) MAGNESIUM STEARATE (UNII: 70097M6I30) ZINC GLUCONATE (UNII: U6WSN5SQ1Z) COPPER GLUCONATE (UNII: RV823G6G67) butylene glycol (UNII: 3XUS85K0RA) mannitol (UNII: 3OWL53L36A) EDETATE SODIUM (UNII: MP1J8420LU) magnesium aspartate (UNII: R17X820ROL) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) phenoxyethanol (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50544-004-91 1 in 1 CARTON 1 NDC:50544-004-02 48 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 09/06/2011 Labeler - University Medical Pharmaceuticals Corp (809706252) Registrant - University Medical Pharmaceuticals Corp (809706252) Establishment Name Address ID/FEI Business Operations Universal Packaging Systems, INC 177711082 manufacture