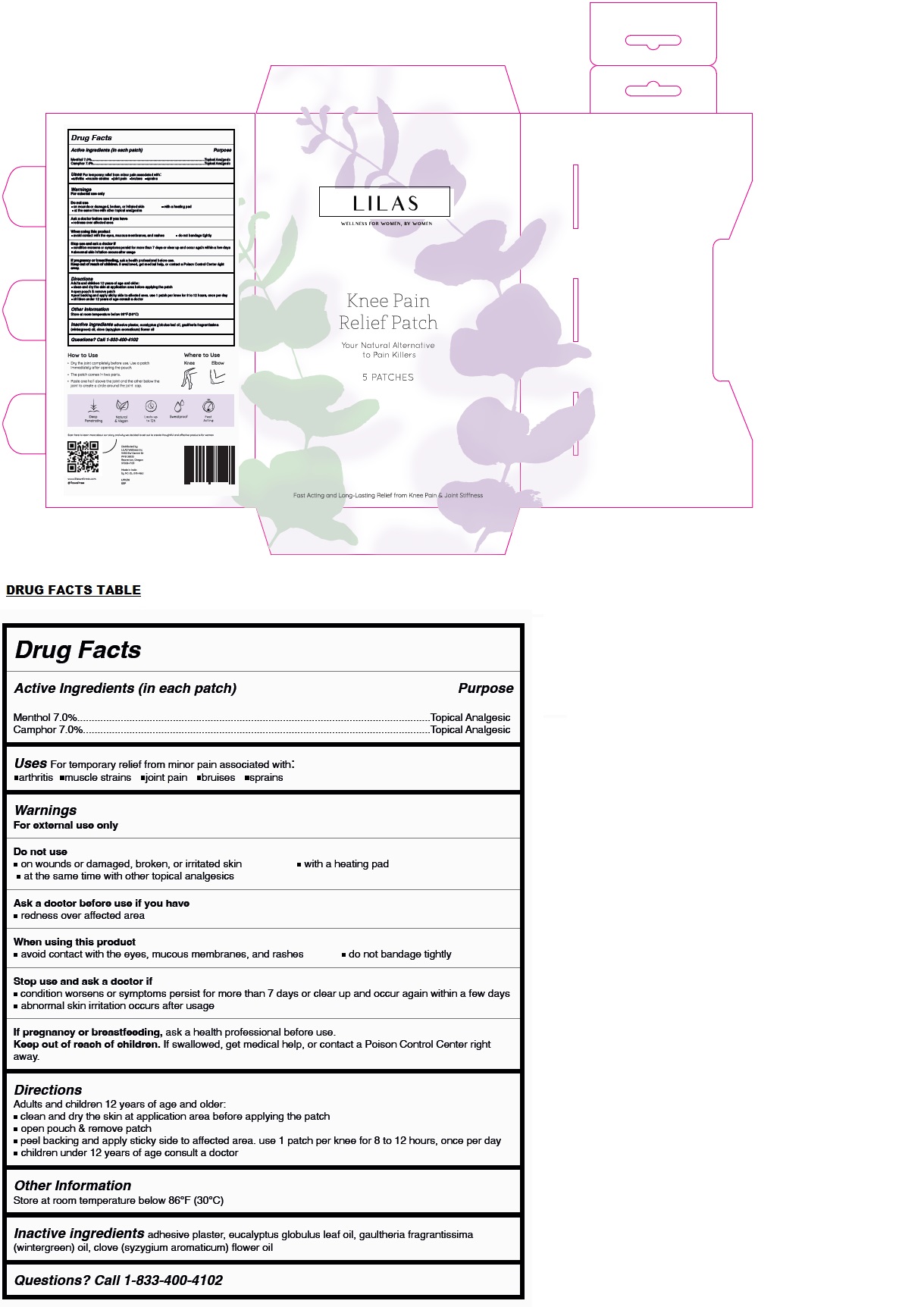
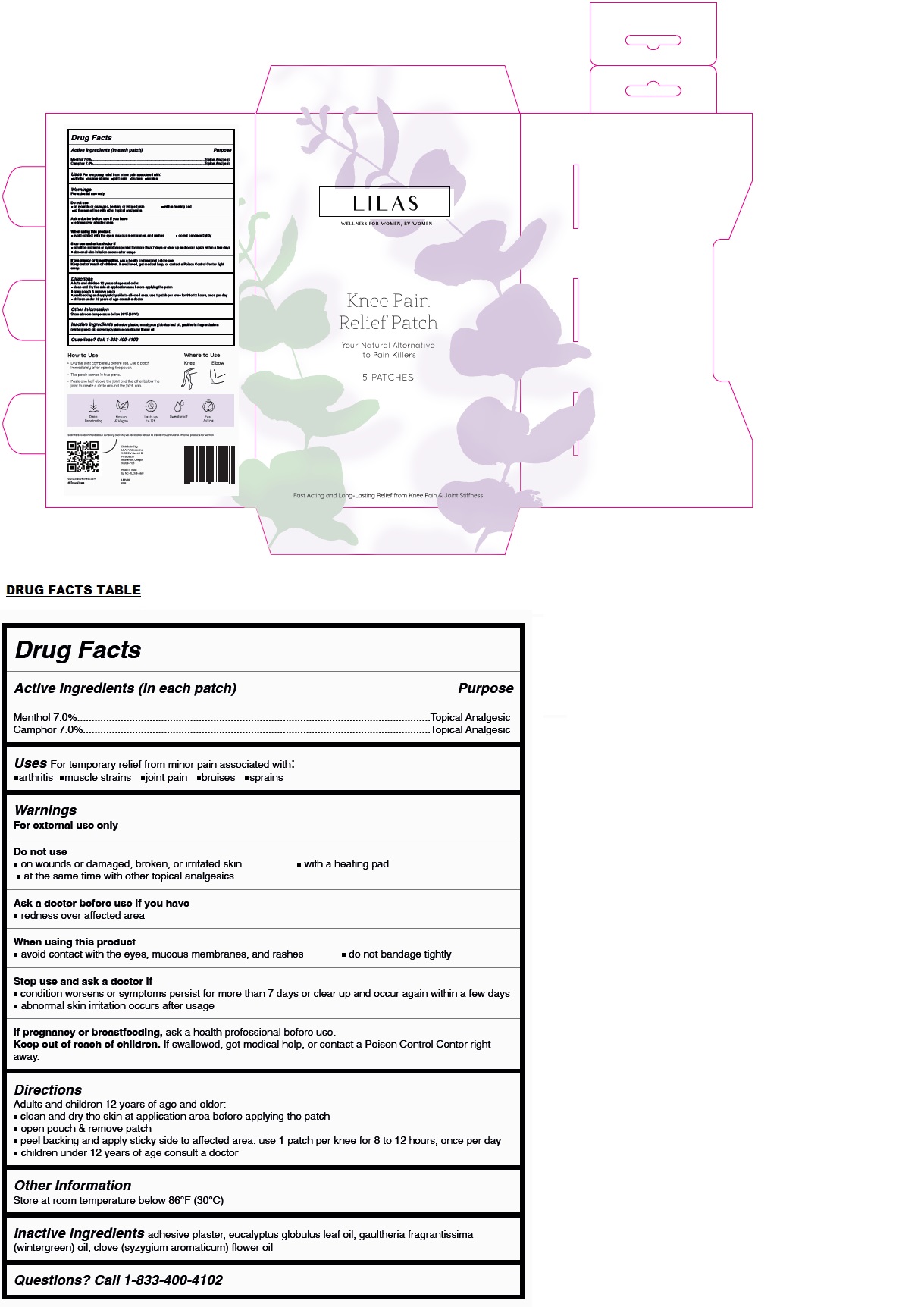
Label: LILAS KNEE PAIN RELIEF PATCH- menthol, camphor patch
- NDC Code(s): 72670-002-05
- Packager: Lilas Wellness, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients (in each patch)
- Purpose
- Uses
-
Warnings
For external use only
Do not use
• on wounds or damaged, broken, or irritated skin • with a heating pad
• at the same time with other topical analgesicsAsk a doctor before use if you have
• redness over affected areaWhen using this product
• avoid contact with the eyes, mucous membranes, and rashes • do not bandage tightlyStop use and ask a doctor if
• condition worsens or symptoms persist for more than 7 days or clear up and occur again within a few days
• abnormal skin irritation occurs after usageIf pregnancy or breastfeeding, ask a health professional before use.
- Directions
- Other Information
- Inactive ingredients
- Questions?
-
SPL UNCLASSIFIED SECTION
WELLNESS FOR WOMEN, BY WOMEN
Your Natural Alternative to Pain Killers
Fast Acting and Long-Lasting Relief from Knee Pain & Joint Stiffness
How to Use
• Dry the joint completely before use. Use a patch immediately after opening the pouch.
• The patch comes in two parts.
• Paste one half above the joint and the other below the joint to create a circle around the joint cap.
Where to Use
Knee Elbow
Deep Penetrating
Natural & Vegan
Lasts up to 12h
Sweatproof
Fast Acting
Distributed by:
LILAS Wellness Inc
9450 SW Gemini Dr
PMB 38333
Beaverton, Oregon
97008-7105Made in India
by NC-DL-378-A&Uwww.lilaswellness.com
@lilaswellness
- Packaging
-
INGREDIENTS AND APPEARANCE
LILAS KNEE PAIN RELIEF PATCH
menthol, camphor patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72670-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 308 mg CAMPHOR (NATURAL) (UNII: N20HL7Q941) (CAMPHOR (NATURAL) - UNII:N20HL7Q941) CAMPHOR (NATURAL) 308 mg Inactive Ingredients Ingredient Name Strength EUCALYPTUS OIL (UNII: 2R04ONI662) METHYL SALICYLATE (UNII: LAV5U5022Y) CLOVE OIL (UNII: 578389D6D0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72670-002-05 5 in 1 PACKAGE 12/12/2023 1 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 12/12/2023 Labeler - Lilas Wellness, Inc. (117655338)