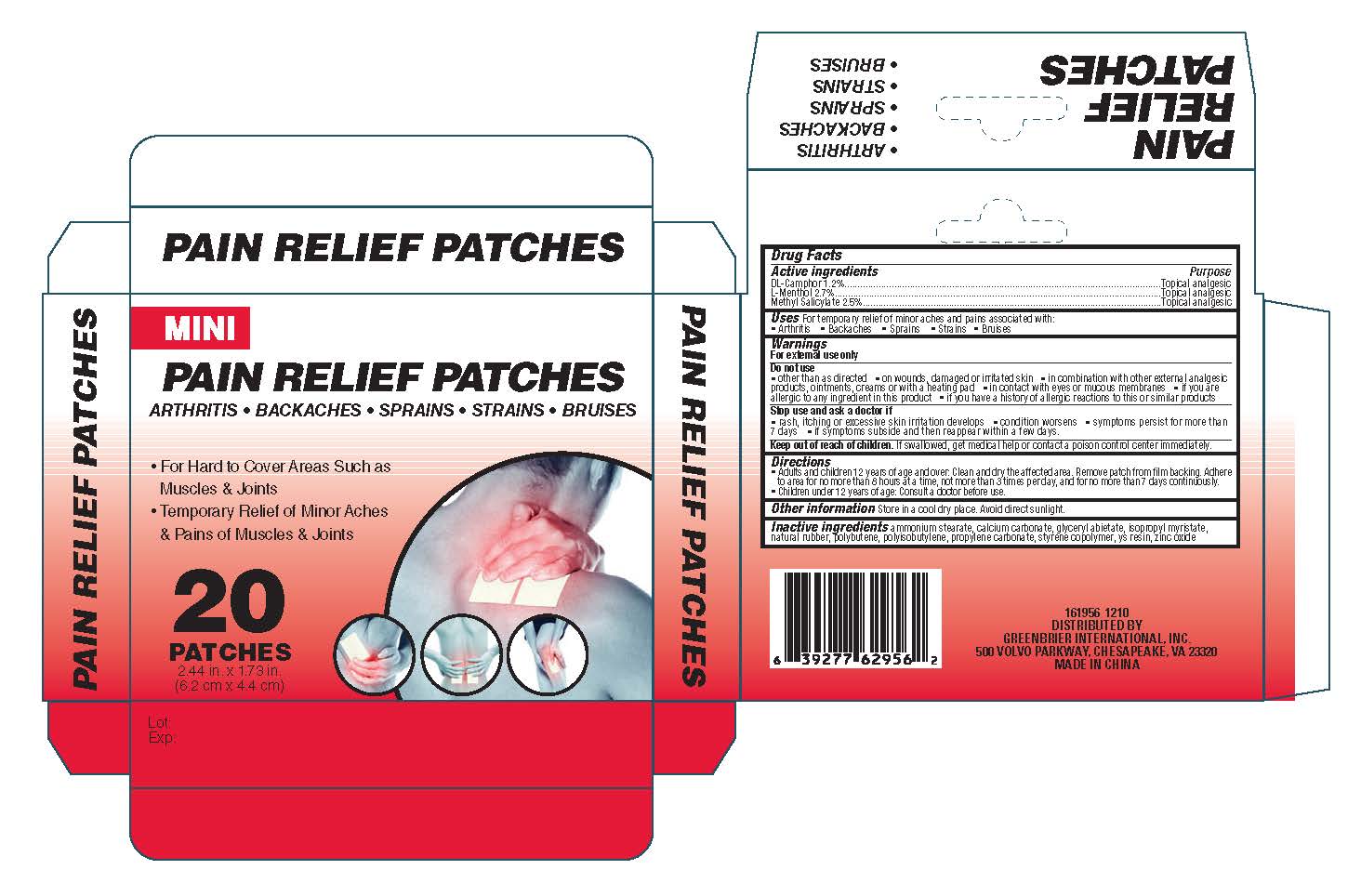
Label: MINI PAIN RELIEF- dl-camphor l-menthol methyl salicylate patch
-
Contains inactivated NDC Code(s)
NDC Code(s): 53043-003-01, 53043-003-02 - Packager: Shanghai Aquagel Bio-Plymer Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 26, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INACTIVE INGREDIENT
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
-
INDICATIONS & USAGE
Do not use
other than as directed on wounds, damaged or irritated skin in combination with other
external analgesic products, ointments, creams or with a heating pad. in contact with eyes or mucous
membranes. if you are allergic to any ingredient in this product or if you have a history of allergic reactions to this
or similar products - STOP USE
- WARNINGS
-
DOSAGE & ADMINISTRATION
Directions
Adults and children 12 years of age and over: Clean and dry the affected area. Remove
patch from film backing. Adhere to area for no more than 8 hours at a time, not more than 3 times per day, and for no more
than 7 days continuously. Children under 12 years of age: Consult a doctor before use. - INSTRUCTIONS FOR USE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MINI PAIN RELIEF
dl-camphor l-menthol methyl salicylate patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53043-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 1.2 mg LEVOMENTHOL (UNII: BZ1R15MTK7) (LEVOMENTHOL - UNII:BZ1R15MTK7) LEVOMENTHOL 2.7 mg METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 2.5 mg Inactive Ingredients Ingredient Name Strength AMMONIUM STEARATE (UNII: 37899330TZ) CALCIUM CARBONATE (UNII: H0G9379FGK) GLYCERYL ABIETATE (UNII: 2F22LY70Q1) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) NATURAL LATEX RUBBER (UNII: 2LQ0UUW8IN) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) POLYISOBUTYLENE (1000 MW) (UNII: 5XB3A63Y52) PROPYLENE CARBONATE (UNII: 8D08K3S51E) STYRENE (UNII: 44LJ2U959V) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53043-003-01 20 in 1 CARTON 2 NDC:53043-003-02 20 in 1 CARTON Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 12/01/2012 Labeler - Shanghai Aquagel Bio-Plymer Co., Ltd (421199544) Establishment Name Address ID/FEI Business Operations Shanghai Aquagel Bio-Plymer Co., Ltd 421199544 manufacture(53043-003, 53043-003)