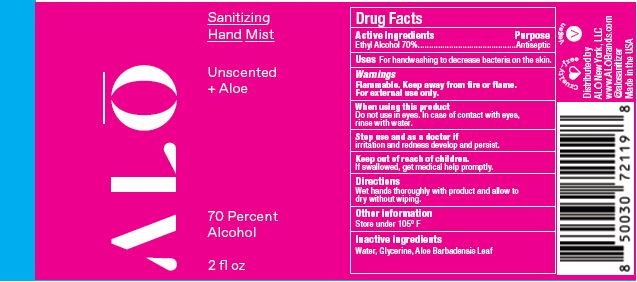
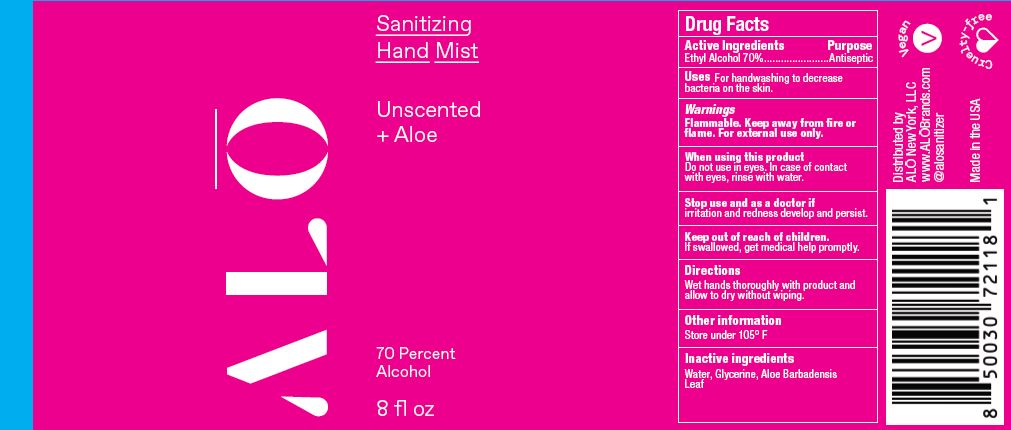
Label: SANITIZING HAND MIST UNSCENTED- ethyl alcohol spray
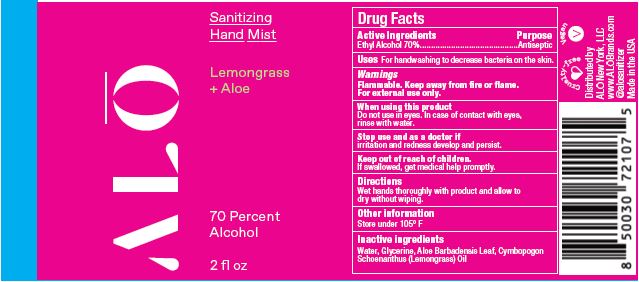
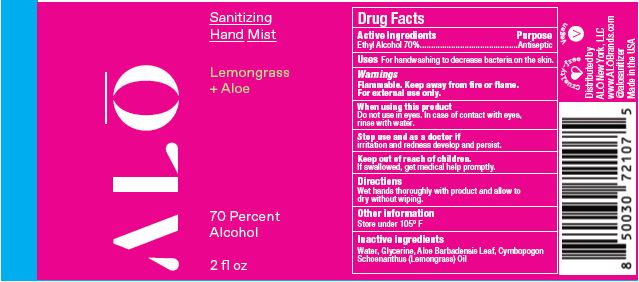
SANITIZING HAND MIST LEMONGRASS- ethyl alcohol spray
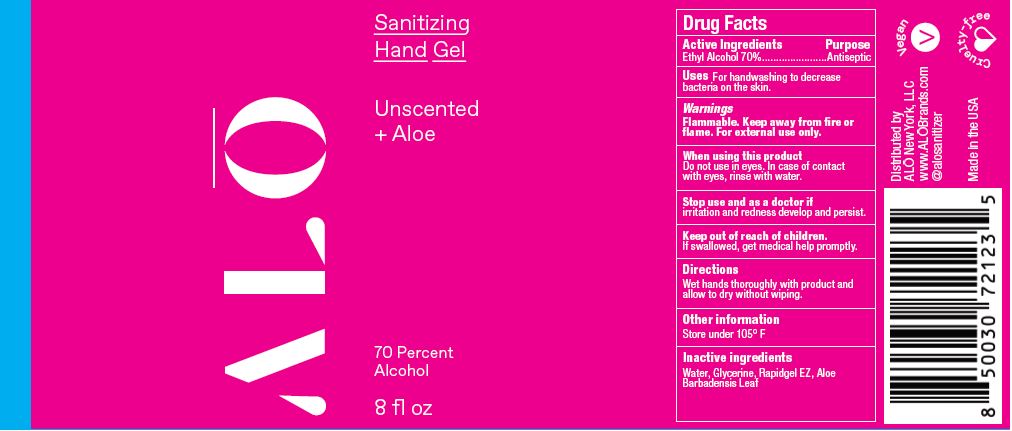
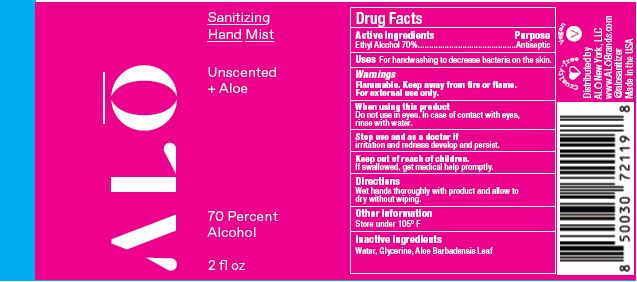
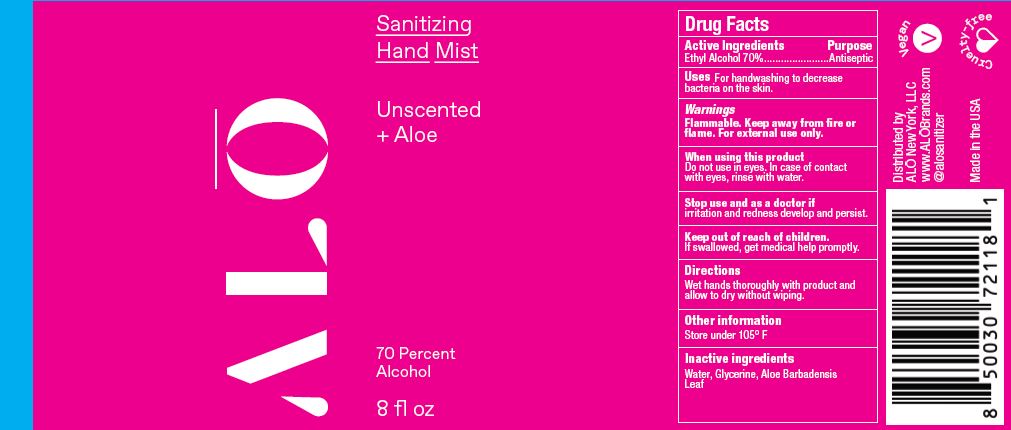
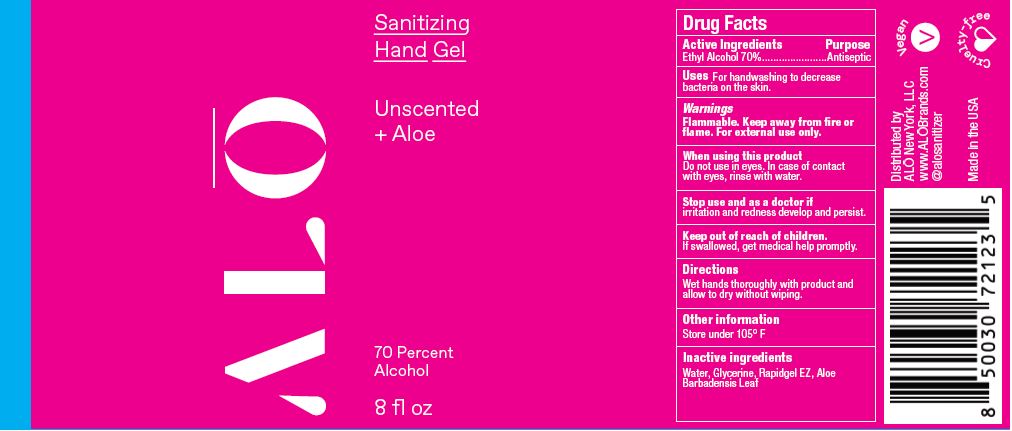
SANITIZING HAND GEL UNSCENTED- ethyl alcohol gel
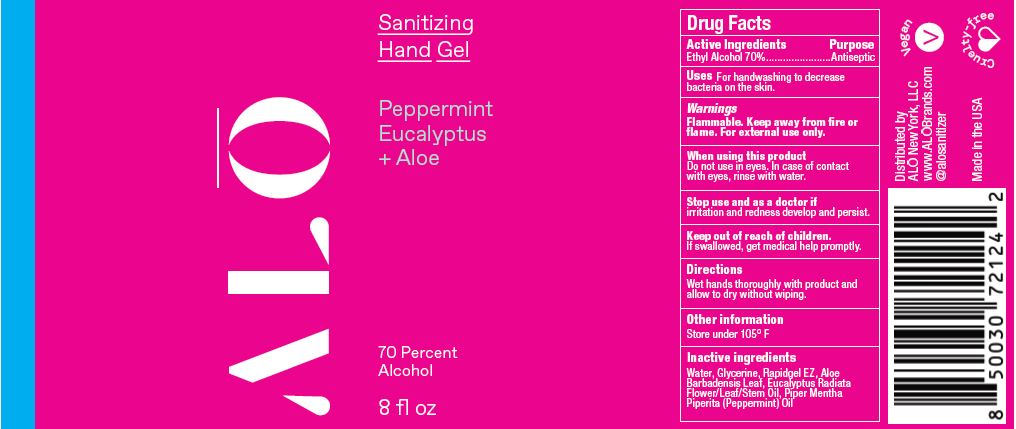
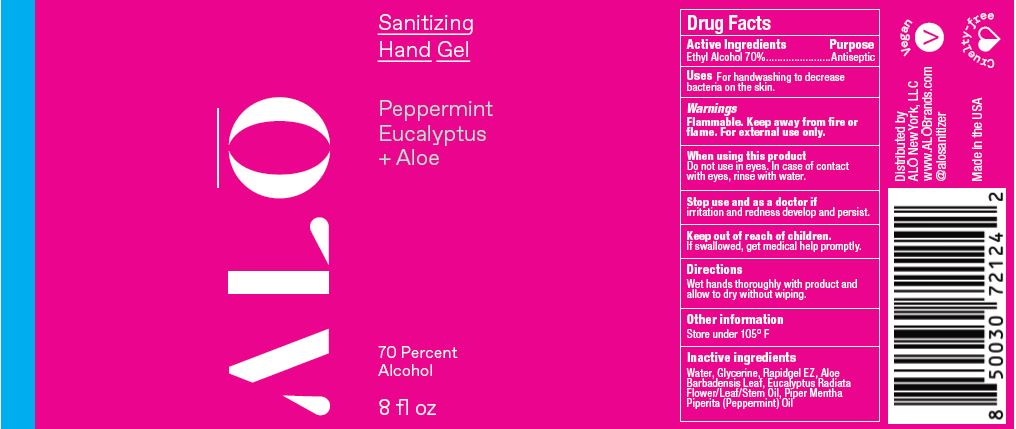
SANITIZING HAND GEL PEPPERMINT EUCALYPTUS- ethyl alcohol gel
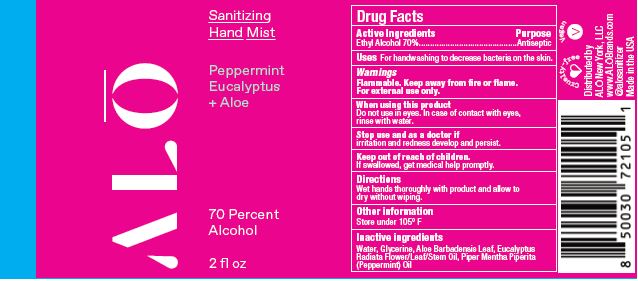
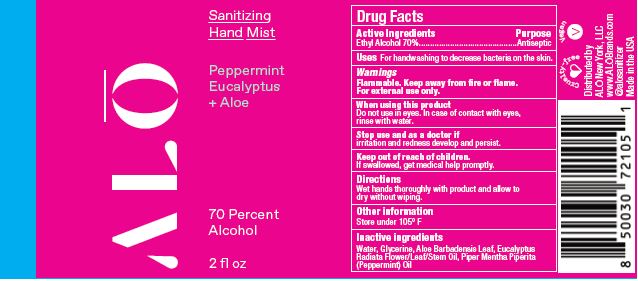
SANITIZING HAND MIST PEPPERMINT EUCALYPTUS- ethyl alcohol spray
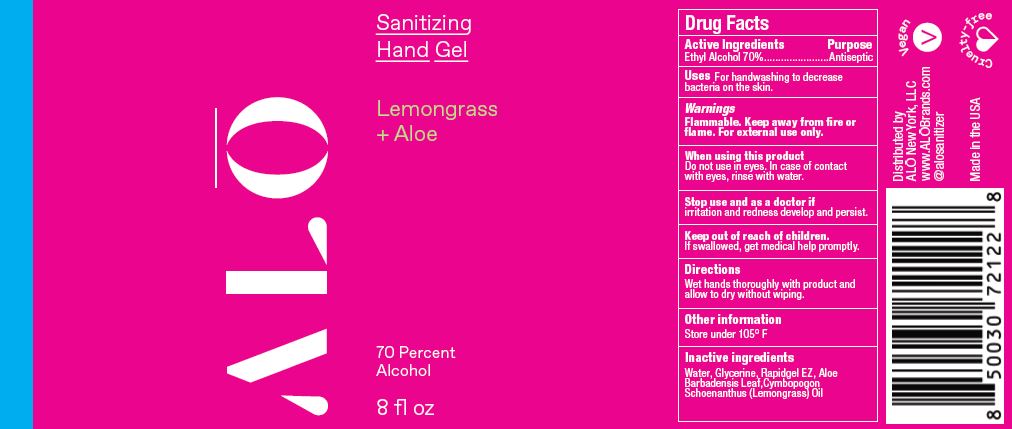
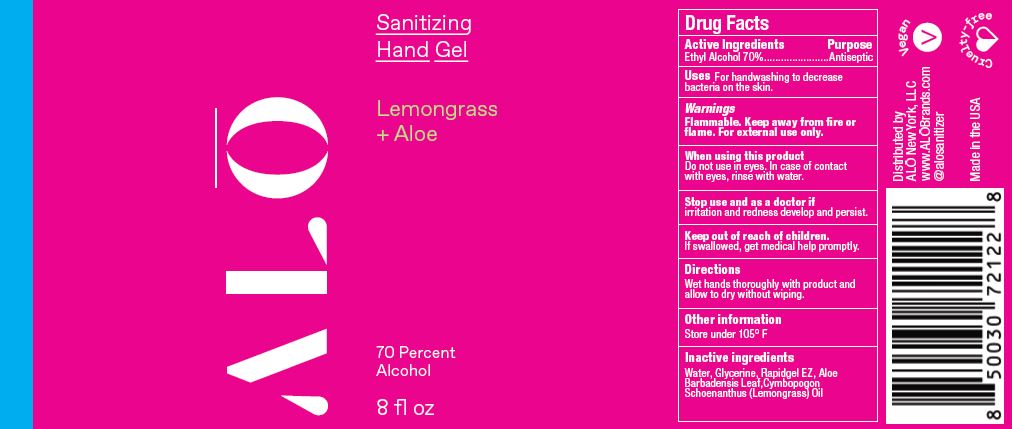
SANITIZING HAND GEL LEMONGRASS- ethyl alcohol gel
-
NDC Code(s):
82355-010-02,
82355-010-08,
82355-011-02,
82355-011-08, view more82355-012-02, 82355-012-08, 82355-020-08, 82355-020-32, 82355-021-08, 82355-021-32, 82355-022-08, 82355-022-32
- Packager: ALO New York LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive Ingredients
NDC 80588-010:
Water, Glycerine, Aloe Barbadensis Leaf
NDC 80588-011:
Water, Glycerine, Alce Barbadensis Leaf, Eucalyptus Radiata Flower/leaf/Stem Oil, Piper Mentha Piperita (Peopermint) OilNDC 80588-012:
Water, Glycerine, Aloe Barbadensis Leaf, Cymbopogon Schoenanthus (Lemongrass) Oil
NDC 80588-020:
Water, Glyperine, Rapidgel EZ. Aloe barbadensis Leaf
NDC 80588-011:
Water. Glycerine, Rapidgel EZ. Aloe Barbadensis Leaf. Eucalyptus Radiate Flower/Leaf/Stem Oil, Piper Mentha Piperita (Peppermint) OilNDC 80588-012:
Water, Glycerine, Rapidgel EZ. Aloe Barbadensis Leaf, Cymbopogon Schoenanthus (Lemongrass) Oil - Sanitizing Hand Mist - Lemongrass +Aloe NDC 82355-012-02
- Sanitizing Hand Gel ReCharge - Peppermint Euclyptus +Aloe NDC 82355-021-32
- Sanitizing Hand Gel ReCharge Lemongrass + Aloe NDC 82355-022-32
- Sanitizing Hand Gel ReCharge - Unscented + Aloe NDC 82355-020-32
- Sanitizing Hand Mist - Peppermint Eucalyptus + Aloe NDC 82355-011-02
- Sanitizing Hand Mist - Unscented + Aloe NDC 82355-010-02
- Sanitizing Hand Mist - Lemongrass +Aloe NDC 82355-012-08
- Sanitizing Hand Mist - Peppermint Eucalypyus + Aloe NDC 82355-011-08
- Sanitizing Hand Mist - Unscented + Aloe NDC 82355-010-08
- Sanitizing Hand Gel - Lemongrass + Aloe NDC 82355-022-08
- Sanitizing Hand Gel - Peppermint Eucalyptus + Aloe NDC 82355-021-08
- Sanitizing Hand Gel - Unscented + Aloe NDC 82355-020-08
-
INGREDIENTS AND APPEARANCE
SANITIZING HAND MIST UNSCENTED
ethyl alcohol sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82355-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength ALOE (UNII: V5VD430YW9) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82355-010-02 59 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 06/09/2022 2 NDC:82355-010-08 236 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 06/09/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 06/09/2022 SANITIZING HAND MIST LEMONGRASS
ethyl alcohol sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82355-012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength ALOE (UNII: V5VD430YW9) EAST INDIAN LEMONGRASS OIL (UNII: UP0M8M3VZW) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82355-012-02 59 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 06/06/2022 2 NDC:82355-012-08 238 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 06/06/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 06/06/2022 SANITIZING HAND GEL UNSCENTED
ethyl alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82355-020 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) ACRYLATES/VINYL ISODECANOATE CROSSPOLYMER (10000 MPA.S NEUTRALIZED AT 0.5%) (UNII: 2N8MDB79NA) WATER (UNII: 059QF0KO0R) ALOE (UNII: V5VD430YW9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82355-020-08 238 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 06/09/2022 2 NDC:82355-020-32 900 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 06/09/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 06/09/2022 SANITIZING HAND GEL PEPPERMINT EUCALYPTUS
ethyl alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82355-021 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength EUCALYPTUS OIL (UNII: 2R04ONI662) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) ACRYLATES/VINYL ISODECANOATE CROSSPOLYMER (10000 MPA.S NEUTRALIZED AT 0.5%) (UNII: 2N8MDB79NA) ALOE (UNII: V5VD430YW9) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PEPPERMINT OIL (UNII: AV092KU4JH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82355-021-08 238 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 06/06/2022 2 NDC:82355-021-32 900 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 06/06/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 06/06/2022 SANITIZING HAND MIST PEPPERMINT EUCALYPTUS
ethyl alcohol sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82355-011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ALOE (UNII: V5VD430YW9) PEPPERMINT OIL (UNII: AV092KU4JH) EUCALYPTUS OIL (UNII: 2R04ONI662) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82355-011-02 59 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 06/06/2022 2 NDC:82355-011-08 238 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 06/06/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 06/06/2022 SANITIZING HAND GEL LEMONGRASS
ethyl alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82355-022 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) ALOE (UNII: V5VD430YW9) EAST INDIAN LEMONGRASS OIL (UNII: UP0M8M3VZW) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) ACRYLATES/VINYL ISODECANOATE CROSSPOLYMER (10000 MPA.S NEUTRALIZED AT 0.5%) (UNII: 2N8MDB79NA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82355-022-08 238 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 06/06/2022 2 NDC:82355-022-32 900 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 06/06/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 06/06/2022 Labeler - ALO New York LLC (110122374) Registrant - Alkaline Corporation (790098318) Establishment Name Address ID/FEI Business Operations Alkaline Corporation 790098318 manufacture(82355-010, 82355-011, 82355-012, 82355-020, 82355-021, 82355-022) , pack(82355-010, 82355-011, 82355-012, 82355-020, 82355-021, 82355-022) , label(82355-010, 82355-011, 82355-012, 82355-020, 82355-021, 82355-022)