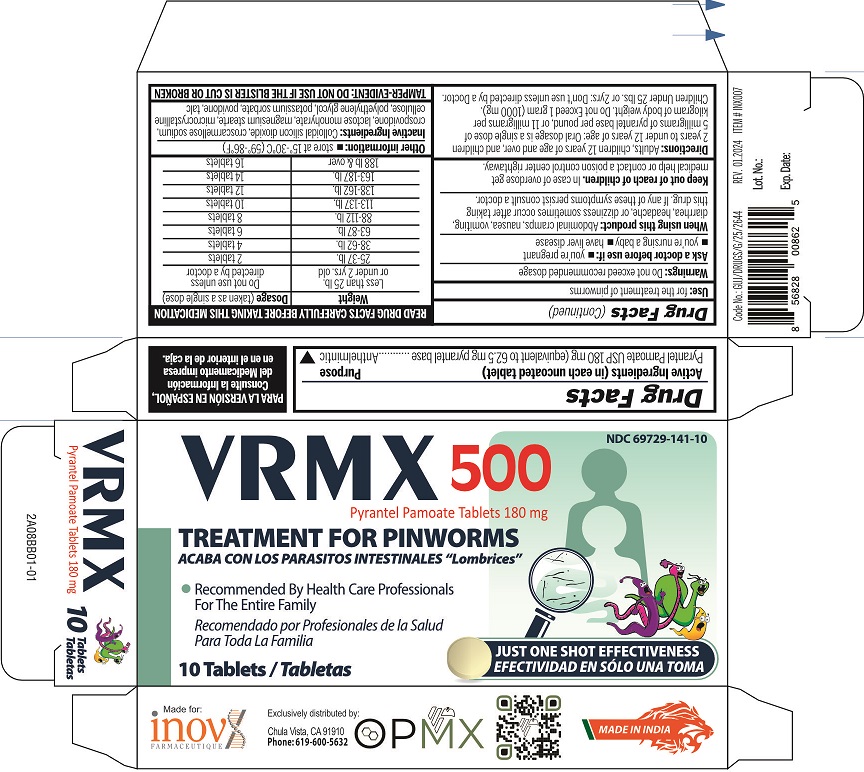
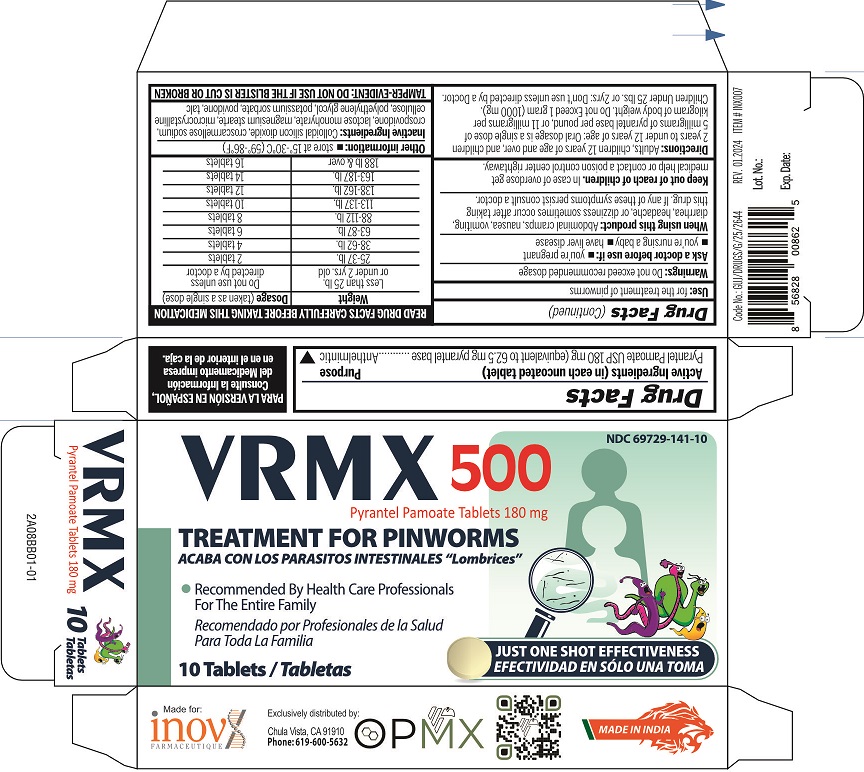
Label: VRMX 500- pyrantel pamoate tablet
- NDC Code(s): 69729-141-10
- Packager: OPMX LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
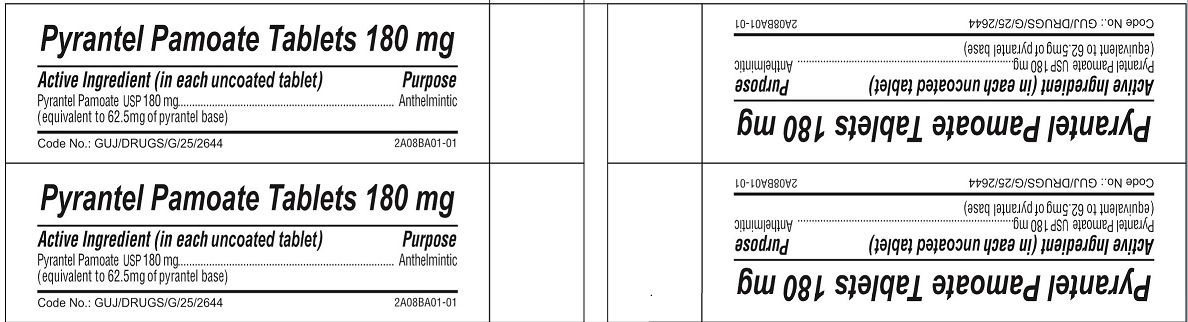
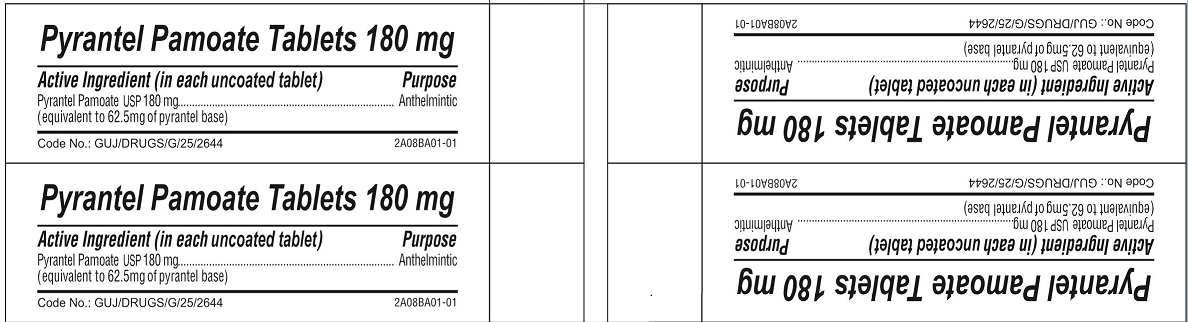
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
Directions:
Adults, children 12 years of age and over, and children 2 years to under 12 years: Oral dossage is a single dose of 5 mg of pyrantel base per pound or 11 mg of pyrantel base per kilogram of body weight. Do not Exceed 1 gram (1000mg). Children Under 25lbs. or 2yrs: Don't use unless directed by a doctor.
READ PACKAGE INSERT CAREFULLY BEFORE TAKING THIS MEDICATION
Weight Dosage (taken as a single dose) Less than 25 lb. or under 2 yrs. old Do not use unless directed by a doctor 25-37 lb. 2 tablets 38-62 lb. 4 tablets 63-87 lb. 6 tablets 88-112 lb. 8 tablets 113-137 lb. 10 tablets 138-162 lb. 12 tablets 163-187 lb. 14 tablets 188 lb & over 16 tablets - STORAGE AND HANDLING
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VRMX 500
pyrantel pamoate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69729-141 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRANTEL PAMOATE (UNII: 81BK194Z5M) (PYRANTEL - UNII:4QIH0N49E7) PYRANTEL 180 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSPOVIDONE (UNII: 2S7830E561) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) TALC (UNII: 7SEV7J4R1U) POLYETHYLENE GLYCOL 6000 (UNII: 30IQX730WE) Product Characteristics Color yellow Score no score Shape ROUND Size 13mm Flavor Imprint Code G Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69729-141-10 1 in 1 CARTON 06/10/2022 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M024 06/10/2022 Labeler - OPMX LLC (029918743)