Label: CERAMIDE LIFT AND FIRM DAY BROAD SPECTRUM SUNSCREEN SPF 30- octinoxate, oxybenzone, octisalate, and avobenzone lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 67938-0802-1, 67938-0802-2 - Packager: Elizabeth Arden, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 19, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
A lightweight, oil-free lotion for face, neck, and decolletage to quickly infuse skin with ceramides for a youth-restoring boost. Absorbs quickly to provide intense hydration throughout the day, leaving skin feeling soft and firm. Diminished the appearance of surface lines and helps restore radiance for a more youthful looking complexion.
-
INDICATIONS AND USAGE
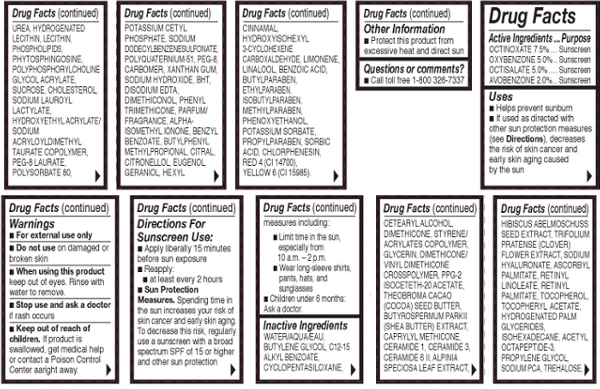
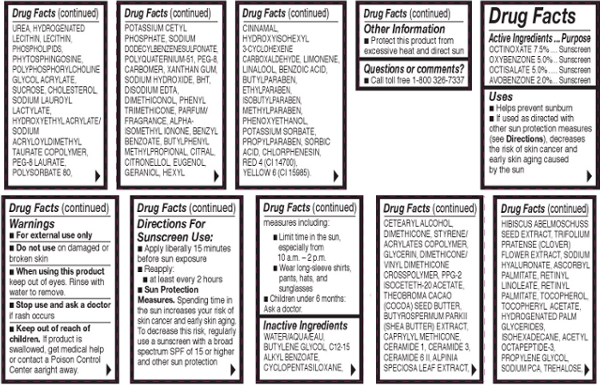
To Use: Apply to cleansed skin after Ceramide Capsules in the morning.
Directions for Sunscreen Use: Apply liberally 15 minutes before sun exposure. Reapply at least every 2 hours.
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including: Limit time in the sun, especially from 10 a.m. - 2 p.m.. Wear long-sleeve shirts, pants, hats, and sunglesses.
- WARNINGS
- OTC - ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Other Ingredients: Water/Aqua/Eau, Butylene Glycol, C12-15 Alkyl Benzoate, Cyclopentasiloxane, Cetearyl Alcohol, Dimethicone, Styrene/Acrylates Copolymer, Glycerin, Dimethicone/Vinyl Dimethicone Crosspolymer, PPG-2 Isoceteth-20 Acetate, Theobroma Cacao (Cocoa) Seed Butter, Butyrospermum Parkii (Shea Butter) Extract, Caprylyl Methicone, Ceramide 1, Ceramide 3, Ceramide 6 II, Alpinia Speciosa Leaf Extract, Hibiscus Abeloschuss Seed Extract, Trifolium Pratense (Clover) Flower Extract, Sodium Hyaluronate, Ascorbyl Palmitate, Retinyl Linoleate, Retinyl Palmitate, Tocopherol, Tocopheryl Acetate, Hydrogenated Lecithin, Lecithin, Phospholipids, Phytosphingosine, Polyphosphorylcholine Glycol Acrylate, Sucrose, Cholesterol, Sodium Lauroyl Lactylate, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, PEG-8 Laurate, Polysorbate 80, Potassium Cetyl Phosphate, Sodium Dodecylbenzenesulfonate, Polyquaternium-51, PEG-8, Carbomer, Xanthan Gum, Sodium Hydroxide, BHT, Disodium EDTA, Dimethiconol, Phenyl Trimethicone, Parfum/Fragrance, Alpha-Isomethyl Ionone, Benzyl Benzoate, Butylphenyl Methylpropional Citral, Citronellol, Eugenol, Geraniol, Hexyl Cinnamai, Hydroxyisohexyl 3-Cyclohexene, Carboxaldehyde Limonene, Linalool, Benzoic Acid, Butylparaben, Ethylparaben, Isobutylparaben, Methylparaben, Phenoxyethanol, Potassium Sorbate, Propylparaben, Sorbic Acid, Chlorphenesin, Red 4 (CI 14700), Yellow 6 (CI 15985).
- DOSAGE & ADMINISTRATION
- OTC - KEEP OUT OF REACH OF CHILDREN
- OTC - PURPOSE
- OTC - WHEN USING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CERAMIDE LIFT AND FIRM DAY BROAD SPECTRUM SUNSCREEN SPF 30
octinoxate, oxybenzone, octisalate, and avobenzone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67938-0802 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 3.75 g in 50 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 2.5 g in 50 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 2.5 g in 50 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 1 g in 50 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) COCOA BUTTER (UNII: 512OYT1CRR) SHEA BUTTER (UNII: K49155WL9Y) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) CERAMIDE 1 (UNII: 5THT33P7X7) CERAMIDE 3 (UNII: 4370DF050B) CERAMIDE 6 II (UNII: F1X8L2B00J) ALPINIA ZERUMBET LEAF (UNII: MS8P33AMKX) ABELMOSCHUS MOSCHATUS SEED (UNII: UN2QZ55I88) TRIFOLIUM PRATENSE FLOWER (UNII: 4JS0838828) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ASCORBYL PALMITATE (UNII: QN83US2B0N) RETINYL LINOLEATE (UNII: 61911N8D6W) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) TOCOPHEROL (UNII: R0ZB2556P8) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) HYDROGENATED PALM GLYCERIDES (UNII: YCZ8EM144Q) ISOHEXADECANE (UNII: 918X1OUF1E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) TREHALOSE (UNII: B8WCK70T7I) UREA (UNII: 8W8T17847W) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) OMEGA-3 FATTY ACIDS (UNII: 71M78END5S) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) SUCROSE (UNII: C151H8M554) CHOLESTEROL (UNII: 97C5T2UQ7J) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) PEG-8 LAURATE (UNII: 762O8IWA10) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) SODIUM DODECYLBENZENESULFONATE (UNII: 554127163Y) XANTHAN GUM (UNII: TTV12P4NEE) SODIUM HYDROXIDE (UNII: 55X04QC32I) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) BENZYL BENZOATE (UNII: N863NB338G) BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) CITRAL (UNII: T7EU0O9VPP) EUGENOL (UNII: 3T8H1794QW) GERANIOL (UNII: L837108USY) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) HYDROXYISOHEXYL 3-CYCLOHEXENE CARBOXALDEHYDE (UNII: QUE43B9Z2Q) BENZOIC ACID (UNII: 8SKN0B0MIM) BUTYLPARABEN (UNII: 3QPI1U3FV8) ETHYLPARABEN (UNII: 14255EXE39) ISOBUTYLPARABEN (UNII: 0QQJ25X58G) METHYLPARABEN (UNII: A2I8C7HI9T) PHENOXYETHANOL (UNII: HIE492ZZ3T) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PROPYLPARABEN (UNII: Z8IX2SC1OH) SORBIC ACID (UNII: X045WJ989B) CHLORPHENESIN (UNII: I670DAL4SZ) FD&C RED NO. 4 (UNII: X3W0AM1JLX) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67938-0802-1 1 in 1 BOX 1 NDC:67938-0802-2 50 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 02/27/2009 Labeler - Elizabeth Arden, Inc (849222187)