Label: NEUTROGENA HEALTHY SKIN LIQUID MAKEUP SPF 20 - CLASSIC IVORY 10- titanium dioxide cream
NEUTROGENA HEALTHY SKIN LIQUID MAKEUP SPF 20 - BUFF 30- titanium dioxide cream
NEUTROGENA HEALTHY SKIN LIQUID MAKEUP SPF 20 - NATURAL BEIGE 60- titanium dioxide cream
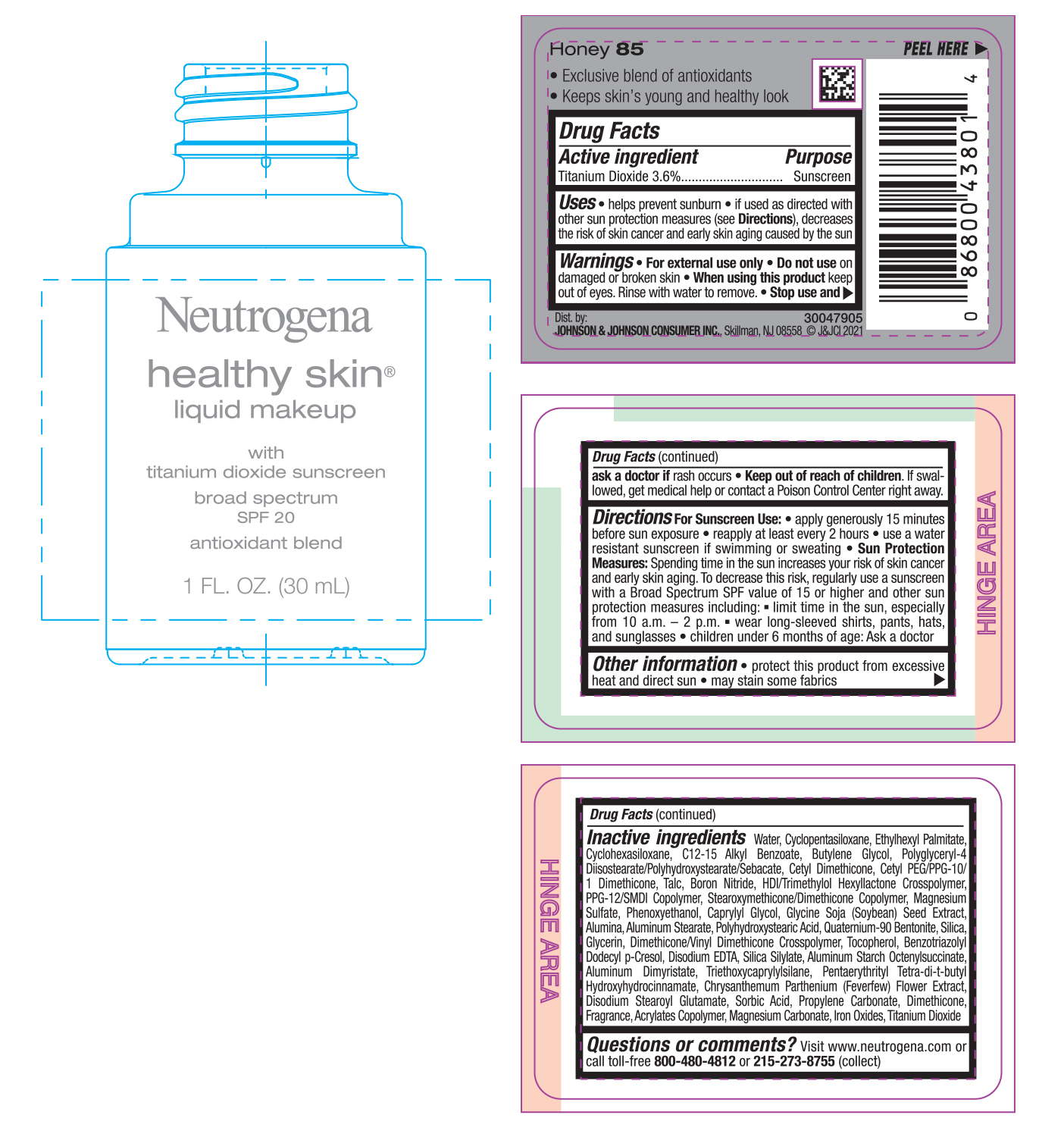
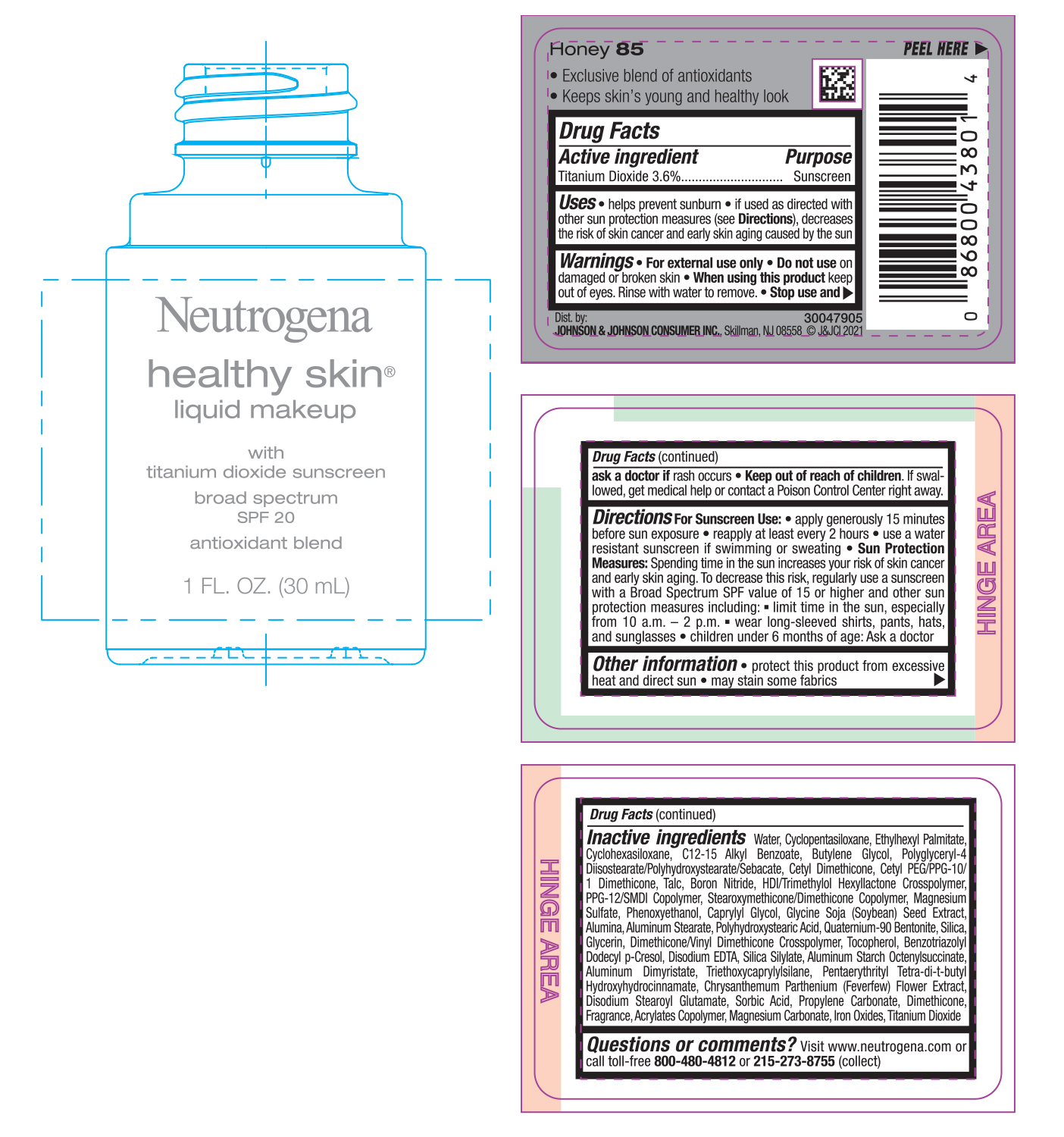
NEUTROGENA HEALTHY SKIN LIQUID MAKEUP SPF 20 - HONEY 85- titanium dioxide cream
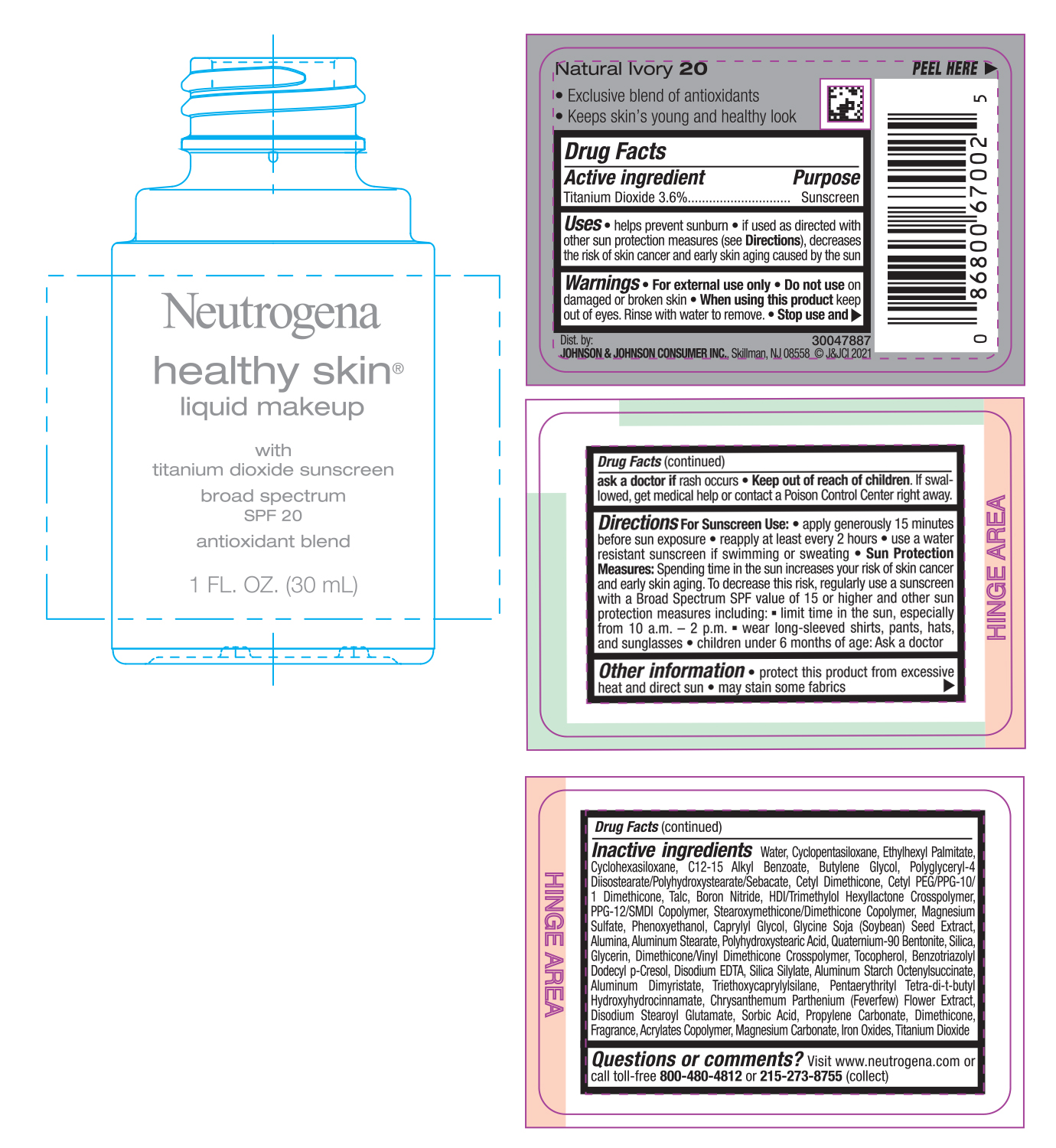
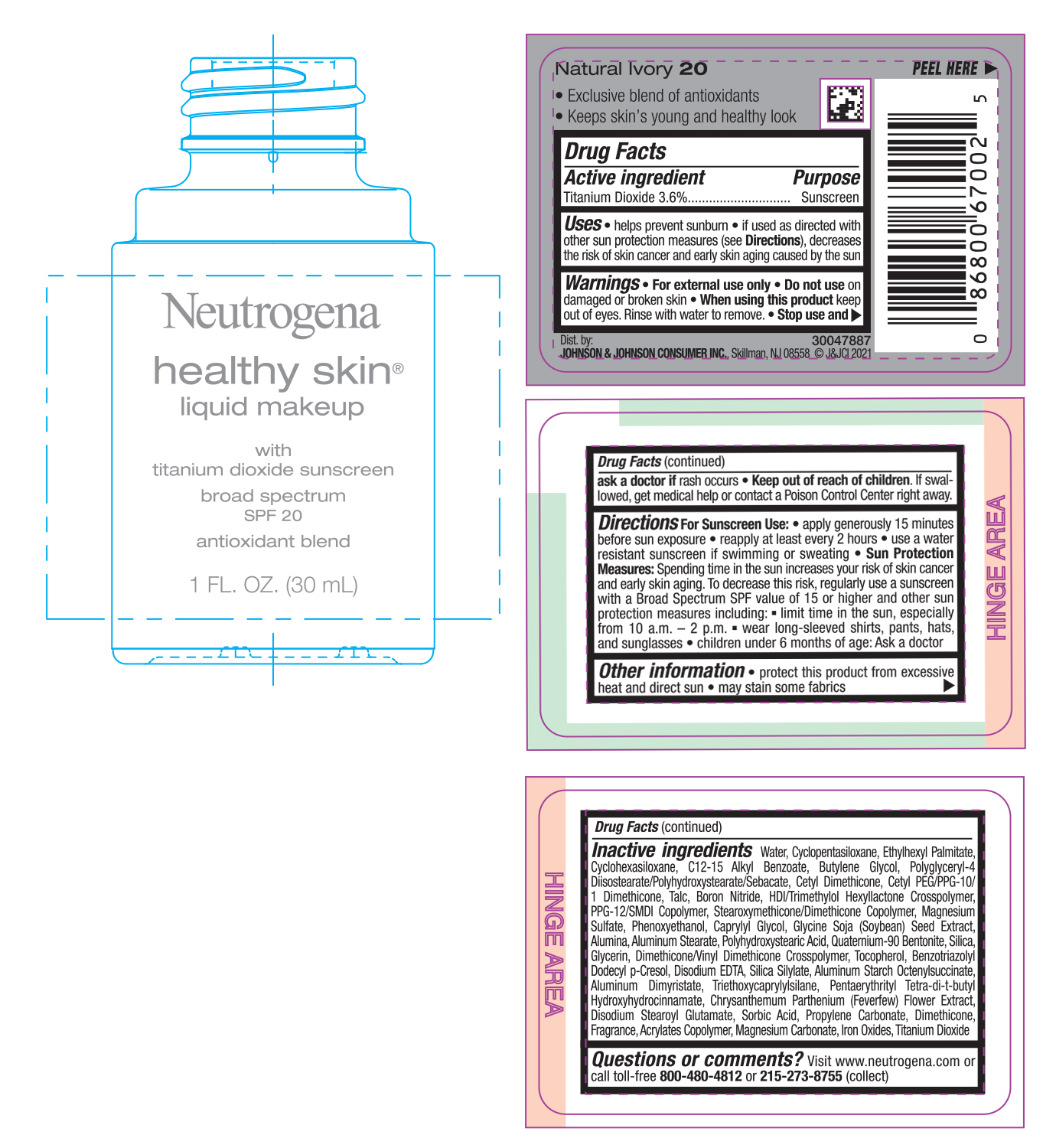
NEUTROGENA HEALTHY SKIN LIQUID MAKEUP SPF 20 - NATURAL IVORY 20- titanium dioxide cream
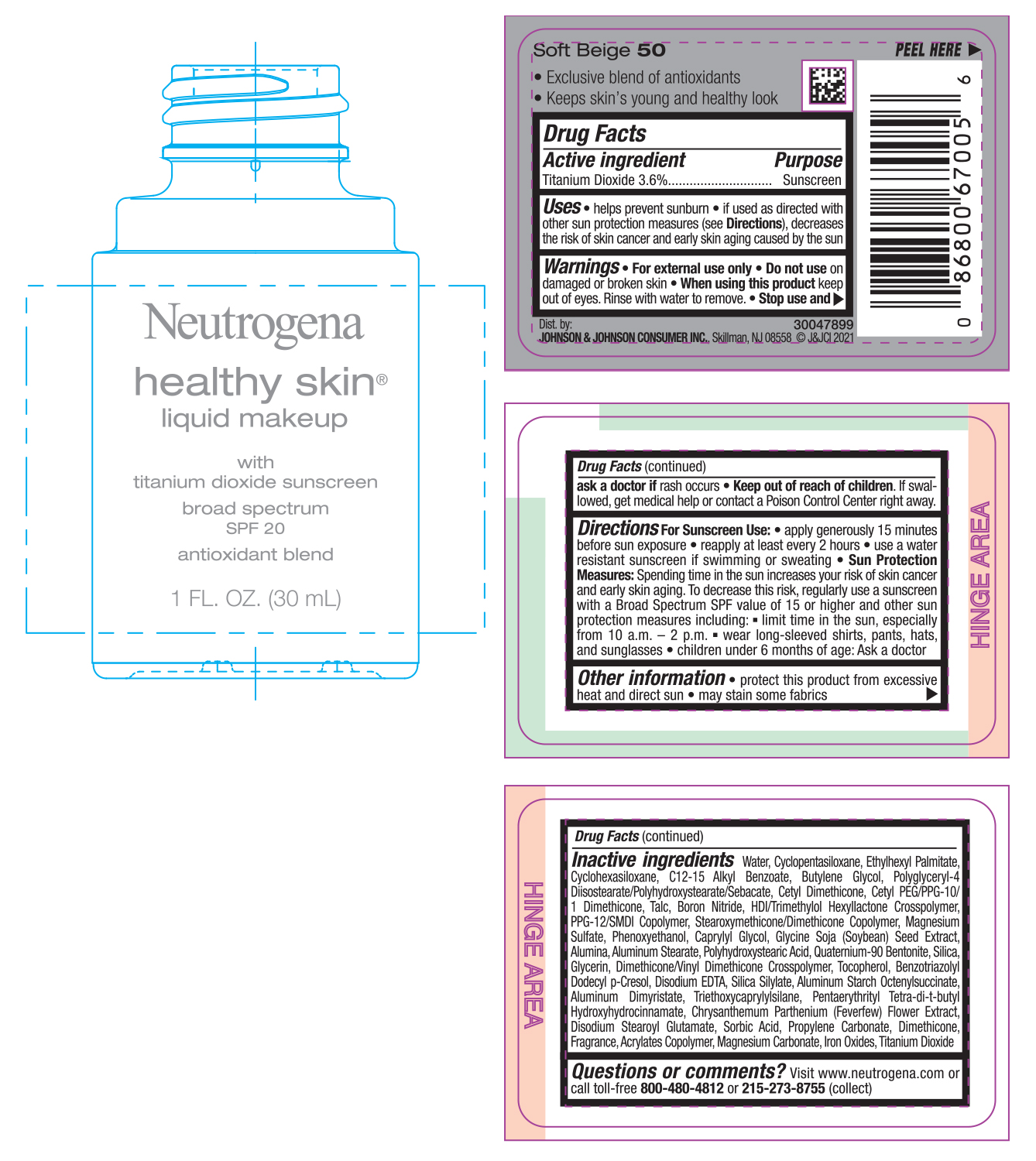
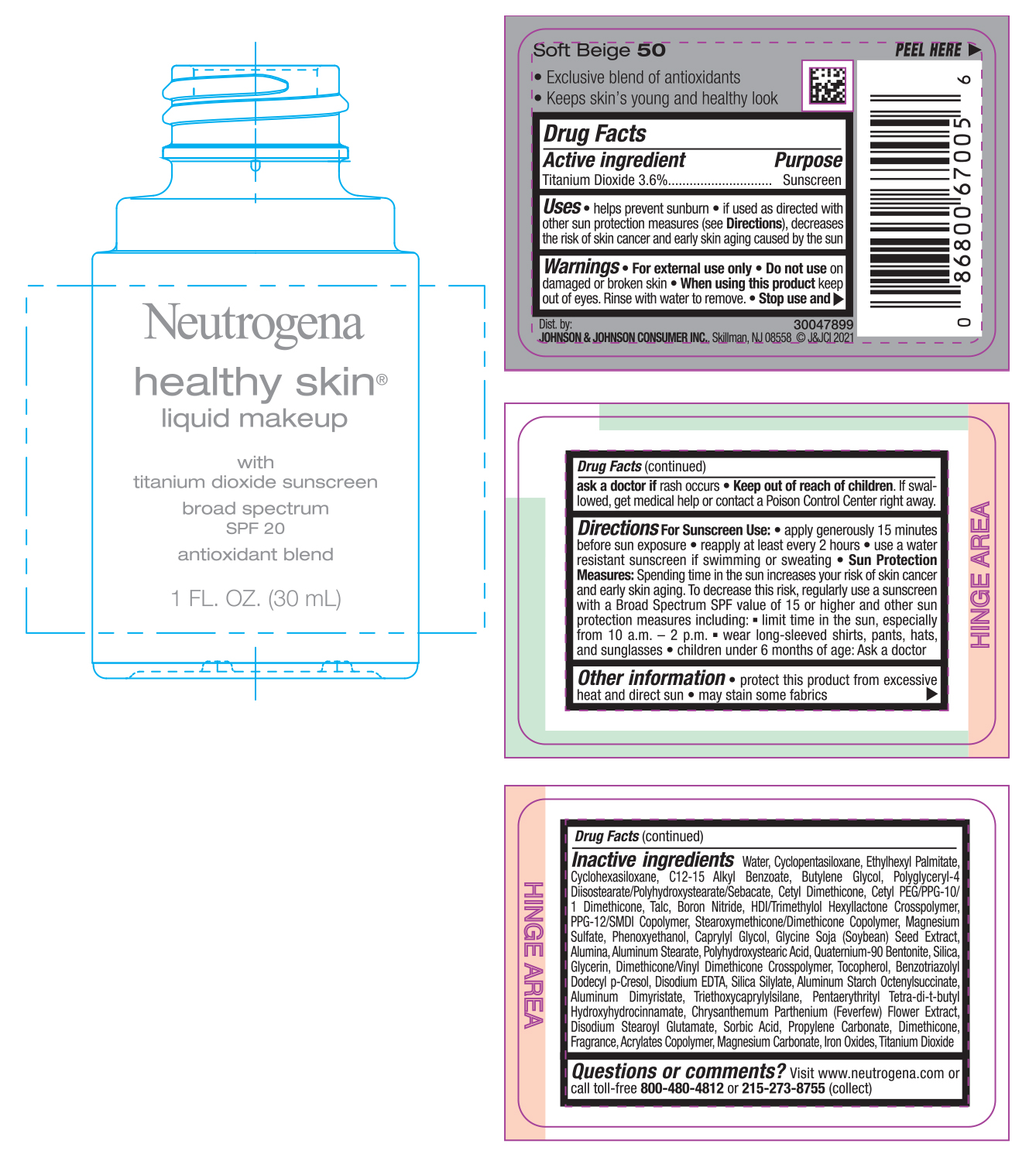
NEUTROGENA HEALTHY SKIN LIQUID MAKEUP SPF 20 - SOFT BEIGE 50- titanium dioxide cream
NEUTROGENA HEALTHY SKIN LIQUID MAKEUP SPF 20 - NUDE 40- titanium dioxide cream
NEUTROGENA HEALTHY SKIN LIQUID MAKEUP SPF 20 - FRESH BEIGE 70- titanium dioxide cream
NEUTROGENA HEALTHY SKIN LIQUID MAKEUP SPF 20 - MEDIUM BEIGE 80- titanium dioxide cream
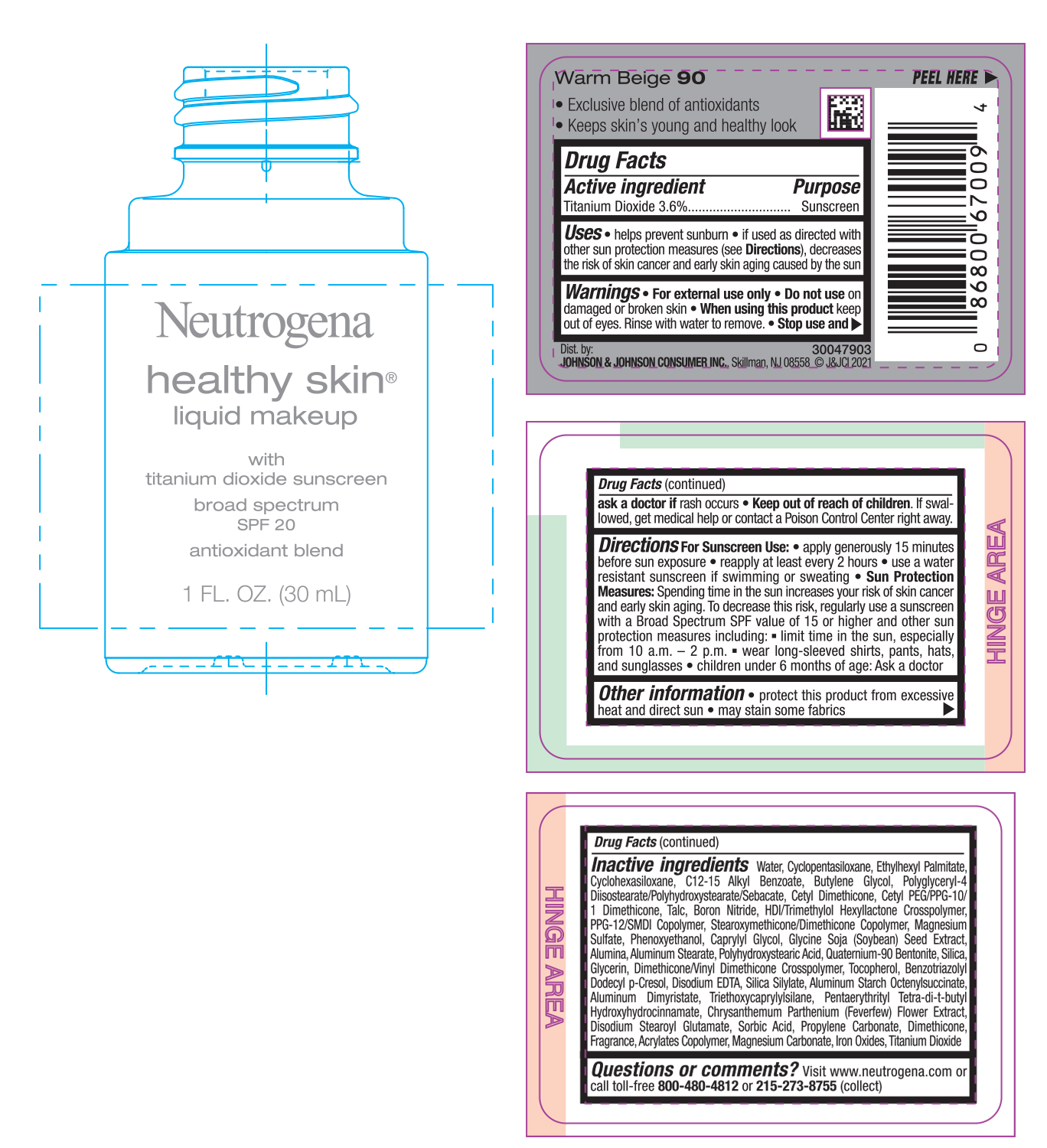
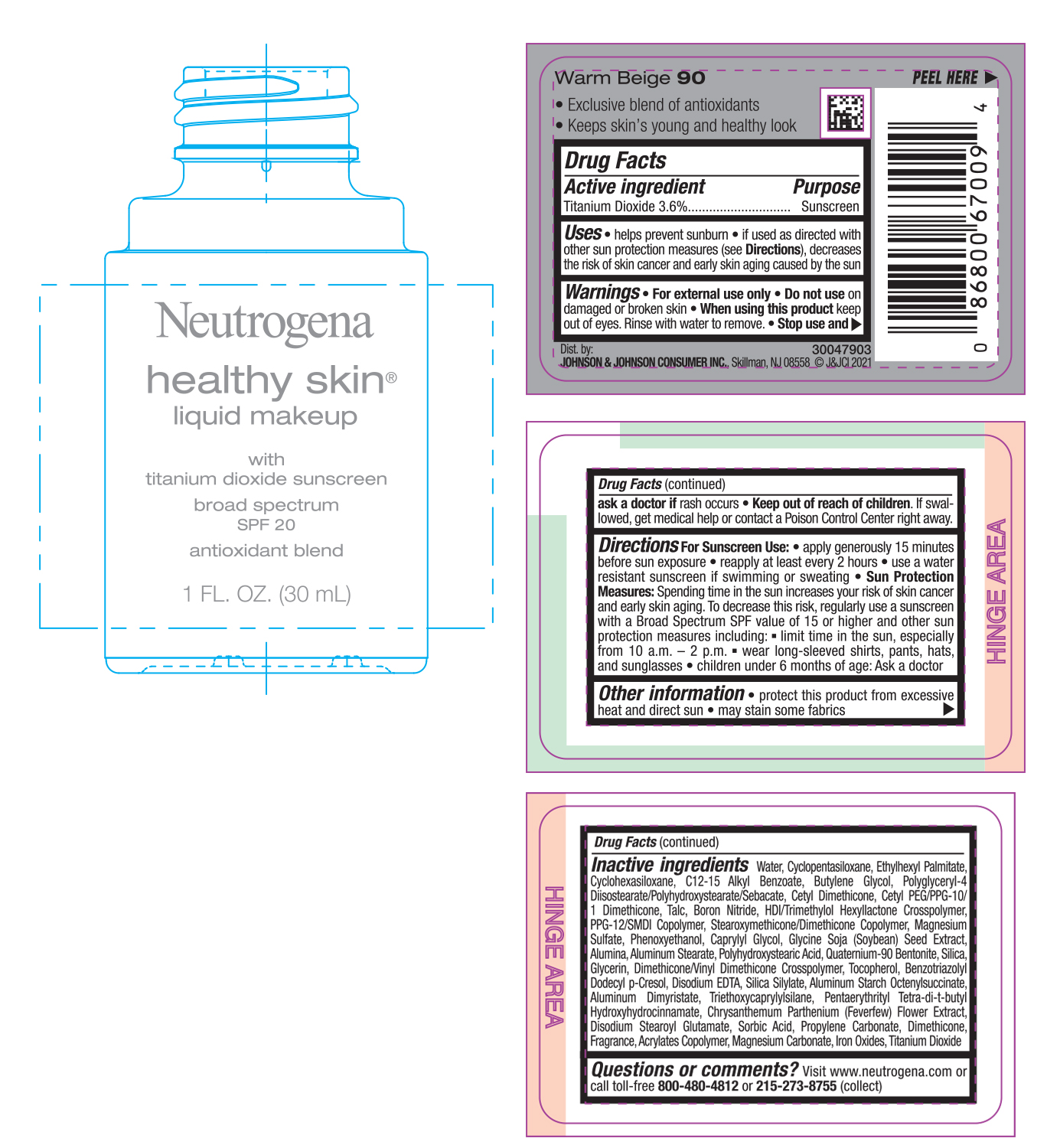
NEUTROGENA HEALTHY SKIN LIQUID MAKEUP SPF 20 - WARM BEIGE 90- titanium dioxide cream
NEUTROGENA HEALTHY SKIN LIQUID MAKEUP SPF 20 - NATURAL TAN 100- titanium dioxide cream
NEUTROGENA HEALTHY SKIN LIQUID MAKEUP SPF 20 - CARAMEL 105- titanium dioxide cream
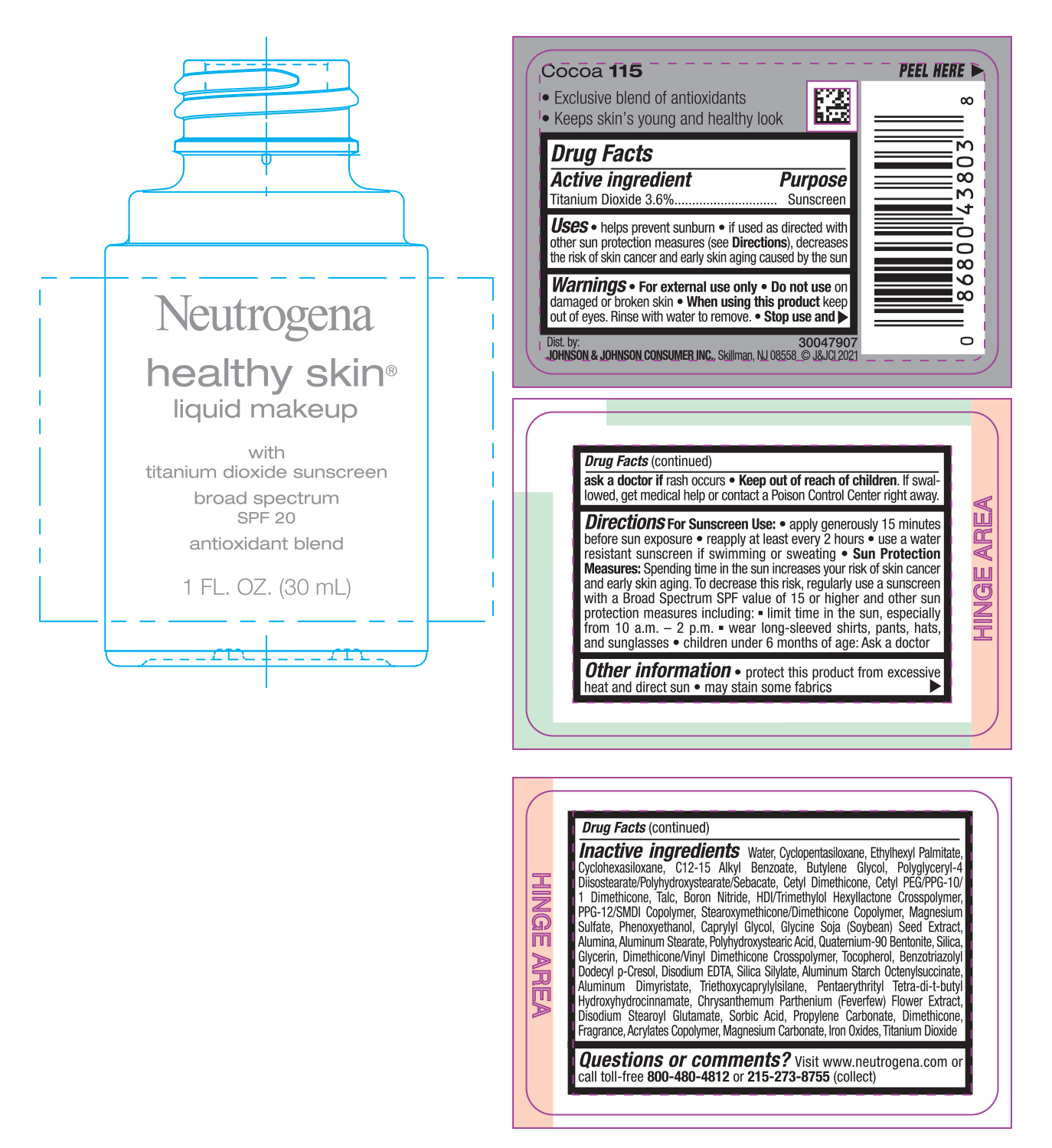
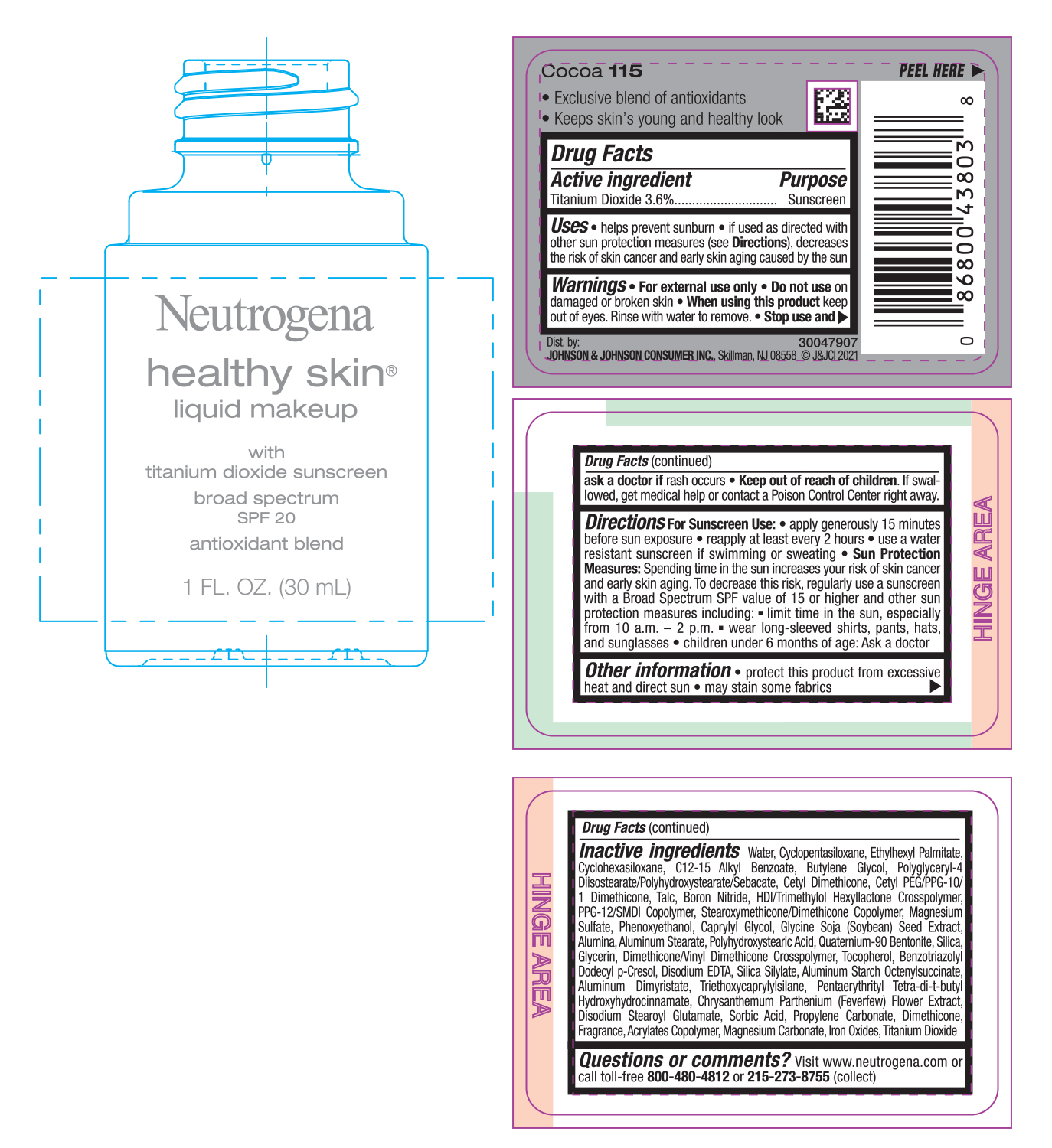
NEUTROGENA HEALTHY SKIN LIQUID MAKEUP SPF 20 - COCOA 115- titanium dioxide cream
NEUTROGENA HEALTHY SKIN LIQUID MAKEUP SPF 20 - CHESTNUT 135- titanium dioxide cream
-
NDC Code(s):
69968-0741-1,
69968-0742-1,
69968-0743-1,
69968-0744-1, view more69968-0745-1, 69968-0746-1, 69968-0747-1, 69968-0748-1, 69968-0749-1, 69968-0750-1, 69968-0751-1, 69968-0752-1, 69968-0753-1, 69968-0754-1
- Packager: Johnson & Johnson Consumer Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
-
Directions
For sunscreen use:
- apply generously 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- children under 6 months of age: Ask a doctor
- Other information
-
Inactive ingredients
Water, Cyclopentasiloxane, Ethylhexyl Palmitate, Cyclohexasiloxane, C12-15 Alkyl Benzoate, Butylene Glycol, Polyglyceryl-4 Diisostearate/Polyhydroxystearate/Sebacate, Cetyl Dimethicone, Cetyl PEG/PPG-10/1 Dimethicone, Talc, Boron Nitride, HDI/Trimethylol Hexyllactone Crosspolymer, PPG-12/SMDI Copolymer, Stearoxymethicone/Dimethicone Copolymer, Magnesium Sulfate, Phenoxyethanol, Caprylyl Glycol, Glycine Soja (Soybean) Seed Extract, Alumina, Aluminum Stearate, Polyhydroxystearic Acid, Quaternium-90 Bentonite, Silica, Glycerin, Dimethicone/Vinyl Dimethicone Crosspolymer, Tocopherol, Benzotriazolyl Dodecyl p-Cresol, Disodium EDTA, Silica Silylate, Aluminum Starch Octenylsuccinate, Aluminum Dimyristate, Triethoxycaprylylsilane, Pentaerythrityl Tetra-di-t-butyl Hydroxyhydrocinnamate, Chrysanthemum Parthenium (Feverfew) Flower Extract, Disodium Stearoyl Glutamate, Sorbic Acid, Propylene Carbonate, Dimethicone, Fragrance, Acrylates Copolymer, Magnesium Carbonate, Iron Oxides, Titanium Dioxide
- Questions or Comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Classic Ivory 10
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Natural Ivory 20
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Buff 30
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Nude 40
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Soft Beige 50
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Natural Beige 60
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Fresh Beige 70
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Medium Beige 80
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Warm Beige 90
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Natural Tan 100
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Honey 85
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Caramel 105
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Cocoa 115
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Chestnut 135
-
INGREDIENTS AND APPEARANCE
NEUTROGENA HEALTHY SKIN LIQUID MAKEUP SPF 20 - CLASSIC IVORY 10
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0741 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL DIMETHICONE 25 (UNII: U4AS1BW4ZB) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) TANACETUM PARTHENIUM FLOWER (UNII: 7TVV9D7I89) ETHYLHEXYL PALMITATE (UNII: 2865993309) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) TALC (UNII: 7SEV7J4R1U) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) SOYBEAN (UNII: L7HT8F1ZOD) ALUMINUM OXIDE (UNII: LMI26O6933) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) GLYCERIN (UNII: PDC6A3C0OX) FERRIC OXIDE RED (UNII: 1K09F3G675) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) TOCOPHEROL (UNII: R0ZB2556P8) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) BORON NITRIDE (UNII: 2U4T60A6YD) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALUMINUM STEARATE (UNII: U6XF9NP8HM) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) SORBIC ACID (UNII: X045WJ989B) PROPYLENE CARBONATE (UNII: 8D08K3S51E) DIMETHICONE (UNII: 92RU3N3Y1O) MAGNESIUM CARBONATE (UNII: 0E53J927NA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) BENZOTRIAZOLYL DODECYL P-CRESOL (UNII: 298PX4M11X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0741-1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/30/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 06/30/2022 NEUTROGENA HEALTHY SKIN LIQUID MAKEUP SPF 20 - BUFF 30
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0743 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength MAGNESIUM CARBONATE (UNII: 0E53J927NA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) BENZOTRIAZOLYL DODECYL P-CRESOL (UNII: 298PX4M11X) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL DIMETHICONE 25 (UNII: U4AS1BW4ZB) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) TANACETUM PARTHENIUM FLOWER (UNII: 7TVV9D7I89) ETHYLHEXYL PALMITATE (UNII: 2865993309) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) TALC (UNII: 7SEV7J4R1U) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) SOYBEAN (UNII: L7HT8F1ZOD) ALUMINUM OXIDE (UNII: LMI26O6933) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) GLYCERIN (UNII: PDC6A3C0OX) FERRIC OXIDE RED (UNII: 1K09F3G675) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) TOCOPHEROL (UNII: R0ZB2556P8) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) BORON NITRIDE (UNII: 2U4T60A6YD) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALUMINUM STEARATE (UNII: U6XF9NP8HM) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) SORBIC ACID (UNII: X045WJ989B) PROPYLENE CARBONATE (UNII: 8D08K3S51E) DIMETHICONE (UNII: 92RU3N3Y1O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0743-1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/30/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 06/30/2022 NEUTROGENA HEALTHY SKIN LIQUID MAKEUP SPF 20 - NATURAL BEIGE 60
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0746 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength MAGNESIUM CARBONATE (UNII: 0E53J927NA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) BENZOTRIAZOLYL DODECYL P-CRESOL (UNII: 298PX4M11X) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL DIMETHICONE 25 (UNII: U4AS1BW4ZB) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) TANACETUM PARTHENIUM FLOWER (UNII: 7TVV9D7I89) ETHYLHEXYL PALMITATE (UNII: 2865993309) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) TALC (UNII: 7SEV7J4R1U) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) SOYBEAN (UNII: L7HT8F1ZOD) ALUMINUM OXIDE (UNII: LMI26O6933) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) GLYCERIN (UNII: PDC6A3C0OX) FERRIC OXIDE RED (UNII: 1K09F3G675) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) TOCOPHEROL (UNII: R0ZB2556P8) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) BORON NITRIDE (UNII: 2U4T60A6YD) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALUMINUM STEARATE (UNII: U6XF9NP8HM) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) SORBIC ACID (UNII: X045WJ989B) PROPYLENE CARBONATE (UNII: 8D08K3S51E) DIMETHICONE (UNII: 92RU3N3Y1O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0746-1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/30/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 06/30/2022 NEUTROGENA HEALTHY SKIN LIQUID MAKEUP SPF 20 - HONEY 85
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0751 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength MAGNESIUM CARBONATE (UNII: 0E53J927NA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) BENZOTRIAZOLYL DODECYL P-CRESOL (UNII: 298PX4M11X) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL DIMETHICONE 25 (UNII: U4AS1BW4ZB) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) TANACETUM PARTHENIUM FLOWER (UNII: 7TVV9D7I89) ETHYLHEXYL PALMITATE (UNII: 2865993309) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) TALC (UNII: 7SEV7J4R1U) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) SOYBEAN (UNII: L7HT8F1ZOD) ALUMINUM OXIDE (UNII: LMI26O6933) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) GLYCERIN (UNII: PDC6A3C0OX) FERRIC OXIDE RED (UNII: 1K09F3G675) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) TOCOPHEROL (UNII: R0ZB2556P8) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) BORON NITRIDE (UNII: 2U4T60A6YD) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALUMINUM STEARATE (UNII: U6XF9NP8HM) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) SORBIC ACID (UNII: X045WJ989B) PROPYLENE CARBONATE (UNII: 8D08K3S51E) DIMETHICONE (UNII: 92RU3N3Y1O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0751-1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/30/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 06/30/2022 NEUTROGENA HEALTHY SKIN LIQUID MAKEUP SPF 20 - NATURAL IVORY 20
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0742 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength MAGNESIUM CARBONATE (UNII: 0E53J927NA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) BENZOTRIAZOLYL DODECYL P-CRESOL (UNII: 298PX4M11X) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL DIMETHICONE 25 (UNII: U4AS1BW4ZB) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) TANACETUM PARTHENIUM FLOWER (UNII: 7TVV9D7I89) ETHYLHEXYL PALMITATE (UNII: 2865993309) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) TALC (UNII: 7SEV7J4R1U) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) SOYBEAN (UNII: L7HT8F1ZOD) ALUMINUM OXIDE (UNII: LMI26O6933) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) GLYCERIN (UNII: PDC6A3C0OX) FERRIC OXIDE RED (UNII: 1K09F3G675) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) TOCOPHEROL (UNII: R0ZB2556P8) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) BORON NITRIDE (UNII: 2U4T60A6YD) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALUMINUM STEARATE (UNII: U6XF9NP8HM) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) SORBIC ACID (UNII: X045WJ989B) PROPYLENE CARBONATE (UNII: 8D08K3S51E) DIMETHICONE (UNII: 92RU3N3Y1O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0742-1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/30/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 06/30/2022 NEUTROGENA HEALTHY SKIN LIQUID MAKEUP SPF 20 - SOFT BEIGE 50
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0745 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength MAGNESIUM CARBONATE (UNII: 0E53J927NA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) BENZOTRIAZOLYL DODECYL P-CRESOL (UNII: 298PX4M11X) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL DIMETHICONE 25 (UNII: U4AS1BW4ZB) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) TANACETUM PARTHENIUM FLOWER (UNII: 7TVV9D7I89) ETHYLHEXYL PALMITATE (UNII: 2865993309) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) TALC (UNII: 7SEV7J4R1U) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) SOYBEAN (UNII: L7HT8F1ZOD) ALUMINUM OXIDE (UNII: LMI26O6933) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) GLYCERIN (UNII: PDC6A3C0OX) FERRIC OXIDE RED (UNII: 1K09F3G675) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) TOCOPHEROL (UNII: R0ZB2556P8) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) BORON NITRIDE (UNII: 2U4T60A6YD) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALUMINUM STEARATE (UNII: U6XF9NP8HM) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) SORBIC ACID (UNII: X045WJ989B) PROPYLENE CARBONATE (UNII: 8D08K3S51E) DIMETHICONE (UNII: 92RU3N3Y1O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0745-1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/30/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 06/30/2022 NEUTROGENA HEALTHY SKIN LIQUID MAKEUP SPF 20 - NUDE 40
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0744 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength MAGNESIUM CARBONATE (UNII: 0E53J927NA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) BENZOTRIAZOLYL DODECYL P-CRESOL (UNII: 298PX4M11X) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL DIMETHICONE 25 (UNII: U4AS1BW4ZB) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) TANACETUM PARTHENIUM FLOWER (UNII: 7TVV9D7I89) ETHYLHEXYL PALMITATE (UNII: 2865993309) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) TALC (UNII: 7SEV7J4R1U) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) SOYBEAN (UNII: L7HT8F1ZOD) ALUMINUM OXIDE (UNII: LMI26O6933) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) GLYCERIN (UNII: PDC6A3C0OX) FERRIC OXIDE RED (UNII: 1K09F3G675) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) TOCOPHEROL (UNII: R0ZB2556P8) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) BORON NITRIDE (UNII: 2U4T60A6YD) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALUMINUM STEARATE (UNII: U6XF9NP8HM) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) SORBIC ACID (UNII: X045WJ989B) PROPYLENE CARBONATE (UNII: 8D08K3S51E) DIMETHICONE (UNII: 92RU3N3Y1O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0744-1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/30/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 06/30/2022 NEUTROGENA HEALTHY SKIN LIQUID MAKEUP SPF 20 - FRESH BEIGE 70
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0747 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength MAGNESIUM CARBONATE (UNII: 0E53J927NA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) BENZOTRIAZOLYL DODECYL P-CRESOL (UNII: 298PX4M11X) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL DIMETHICONE 25 (UNII: U4AS1BW4ZB) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) TANACETUM PARTHENIUM FLOWER (UNII: 7TVV9D7I89) ETHYLHEXYL PALMITATE (UNII: 2865993309) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) TALC (UNII: 7SEV7J4R1U) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) SOYBEAN (UNII: L7HT8F1ZOD) ALUMINUM OXIDE (UNII: LMI26O6933) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) GLYCERIN (UNII: PDC6A3C0OX) FERRIC OXIDE RED (UNII: 1K09F3G675) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) TOCOPHEROL (UNII: R0ZB2556P8) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) BORON NITRIDE (UNII: 2U4T60A6YD) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALUMINUM STEARATE (UNII: U6XF9NP8HM) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) SORBIC ACID (UNII: X045WJ989B) PROPYLENE CARBONATE (UNII: 8D08K3S51E) DIMETHICONE (UNII: 92RU3N3Y1O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0747-1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/30/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 06/30/2022 NEUTROGENA HEALTHY SKIN LIQUID MAKEUP SPF 20 - MEDIUM BEIGE 80
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0748 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength MAGNESIUM CARBONATE (UNII: 0E53J927NA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) BENZOTRIAZOLYL DODECYL P-CRESOL (UNII: 298PX4M11X) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL DIMETHICONE 25 (UNII: U4AS1BW4ZB) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) TANACETUM PARTHENIUM FLOWER (UNII: 7TVV9D7I89) ETHYLHEXYL PALMITATE (UNII: 2865993309) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) TALC (UNII: 7SEV7J4R1U) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) SOYBEAN (UNII: L7HT8F1ZOD) ALUMINUM OXIDE (UNII: LMI26O6933) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) GLYCERIN (UNII: PDC6A3C0OX) FERRIC OXIDE RED (UNII: 1K09F3G675) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) TOCOPHEROL (UNII: R0ZB2556P8) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) BORON NITRIDE (UNII: 2U4T60A6YD) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALUMINUM STEARATE (UNII: U6XF9NP8HM) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) SORBIC ACID (UNII: X045WJ989B) PROPYLENE CARBONATE (UNII: 8D08K3S51E) DIMETHICONE (UNII: 92RU3N3Y1O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0748-1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/30/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 06/30/2022 NEUTROGENA HEALTHY SKIN LIQUID MAKEUP SPF 20 - WARM BEIGE 90
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0749 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength MAGNESIUM CARBONATE (UNII: 0E53J927NA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) BENZOTRIAZOLYL DODECYL P-CRESOL (UNII: 298PX4M11X) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL DIMETHICONE 25 (UNII: U4AS1BW4ZB) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) TANACETUM PARTHENIUM FLOWER (UNII: 7TVV9D7I89) ETHYLHEXYL PALMITATE (UNII: 2865993309) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) TALC (UNII: 7SEV7J4R1U) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) SOYBEAN (UNII: L7HT8F1ZOD) ALUMINUM OXIDE (UNII: LMI26O6933) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) GLYCERIN (UNII: PDC6A3C0OX) FERRIC OXIDE RED (UNII: 1K09F3G675) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) TOCOPHEROL (UNII: R0ZB2556P8) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) BORON NITRIDE (UNII: 2U4T60A6YD) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALUMINUM STEARATE (UNII: U6XF9NP8HM) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) SORBIC ACID (UNII: X045WJ989B) PROPYLENE CARBONATE (UNII: 8D08K3S51E) DIMETHICONE (UNII: 92RU3N3Y1O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0749-1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/30/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 06/30/2022 NEUTROGENA HEALTHY SKIN LIQUID MAKEUP SPF 20 - NATURAL TAN 100
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0750 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength MAGNESIUM CARBONATE (UNII: 0E53J927NA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) BENZOTRIAZOLYL DODECYL P-CRESOL (UNII: 298PX4M11X) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL DIMETHICONE 25 (UNII: U4AS1BW4ZB) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) TANACETUM PARTHENIUM FLOWER (UNII: 7TVV9D7I89) ETHYLHEXYL PALMITATE (UNII: 2865993309) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) TALC (UNII: 7SEV7J4R1U) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) SOYBEAN (UNII: L7HT8F1ZOD) ALUMINUM OXIDE (UNII: LMI26O6933) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) GLYCERIN (UNII: PDC6A3C0OX) FERRIC OXIDE RED (UNII: 1K09F3G675) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) TOCOPHEROL (UNII: R0ZB2556P8) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) BORON NITRIDE (UNII: 2U4T60A6YD) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALUMINUM STEARATE (UNII: U6XF9NP8HM) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) SORBIC ACID (UNII: X045WJ989B) PROPYLENE CARBONATE (UNII: 8D08K3S51E) DIMETHICONE (UNII: 92RU3N3Y1O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0750-1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/30/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 06/30/2022 NEUTROGENA HEALTHY SKIN LIQUID MAKEUP SPF 20 - CARAMEL 105
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0752 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength MAGNESIUM CARBONATE (UNII: 0E53J927NA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) BENZOTRIAZOLYL DODECYL P-CRESOL (UNII: 298PX4M11X) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL DIMETHICONE 25 (UNII: U4AS1BW4ZB) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) TANACETUM PARTHENIUM FLOWER (UNII: 7TVV9D7I89) ETHYLHEXYL PALMITATE (UNII: 2865993309) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) TALC (UNII: 7SEV7J4R1U) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) SOYBEAN (UNII: L7HT8F1ZOD) ALUMINUM OXIDE (UNII: LMI26O6933) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) GLYCERIN (UNII: PDC6A3C0OX) FERRIC OXIDE RED (UNII: 1K09F3G675) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) TOCOPHEROL (UNII: R0ZB2556P8) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) BORON NITRIDE (UNII: 2U4T60A6YD) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALUMINUM STEARATE (UNII: U6XF9NP8HM) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) SORBIC ACID (UNII: X045WJ989B) PROPYLENE CARBONATE (UNII: 8D08K3S51E) DIMETHICONE (UNII: 92RU3N3Y1O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0752-1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/30/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 06/30/2022 NEUTROGENA HEALTHY SKIN LIQUID MAKEUP SPF 20 - COCOA 115
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0753 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength MAGNESIUM CARBONATE (UNII: 0E53J927NA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) BENZOTRIAZOLYL DODECYL P-CRESOL (UNII: 298PX4M11X) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL DIMETHICONE 25 (UNII: U4AS1BW4ZB) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) TANACETUM PARTHENIUM FLOWER (UNII: 7TVV9D7I89) ETHYLHEXYL PALMITATE (UNII: 2865993309) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) TALC (UNII: 7SEV7J4R1U) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) SOYBEAN (UNII: L7HT8F1ZOD) ALUMINUM OXIDE (UNII: LMI26O6933) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) GLYCERIN (UNII: PDC6A3C0OX) FERRIC OXIDE RED (UNII: 1K09F3G675) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) TOCOPHEROL (UNII: R0ZB2556P8) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) BORON NITRIDE (UNII: 2U4T60A6YD) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALUMINUM STEARATE (UNII: U6XF9NP8HM) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) SORBIC ACID (UNII: X045WJ989B) PROPYLENE CARBONATE (UNII: 8D08K3S51E) DIMETHICONE (UNII: 92RU3N3Y1O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0753-1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/30/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 06/30/2022 NEUTROGENA HEALTHY SKIN LIQUID MAKEUP SPF 20 - CHESTNUT 135
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0754 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength MAGNESIUM CARBONATE (UNII: 0E53J927NA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) BENZOTRIAZOLYL DODECYL P-CRESOL (UNII: 298PX4M11X) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL DIMETHICONE 25 (UNII: U4AS1BW4ZB) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) TANACETUM PARTHENIUM FLOWER (UNII: 7TVV9D7I89) ETHYLHEXYL PALMITATE (UNII: 2865993309) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) TALC (UNII: 7SEV7J4R1U) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) SOYBEAN (UNII: L7HT8F1ZOD) ALUMINUM OXIDE (UNII: LMI26O6933) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) GLYCERIN (UNII: PDC6A3C0OX) FERRIC OXIDE RED (UNII: 1K09F3G675) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) TOCOPHEROL (UNII: R0ZB2556P8) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) BORON NITRIDE (UNII: 2U4T60A6YD) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALUMINUM STEARATE (UNII: U6XF9NP8HM) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) SORBIC ACID (UNII: X045WJ989B) PROPYLENE CARBONATE (UNII: 8D08K3S51E) DIMETHICONE (UNII: 92RU3N3Y1O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0754-1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/30/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 06/30/2022 Labeler - Johnson & Johnson Consumer Inc. (118772437)