Label: BUSCAPINA- acetaminophen, caffeine, pyrilamine maleate tablet, coated
- NDC Code(s): 69729-131-50, 69729-131-72
- Packager: OPMX LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Liver Warning:
This product contains acetaminophen. Sever liber damage may occur if you take:
- more than 6 caplets in 24 hours, which is the maximum daily ammount for this product.
- wtih other drugs containing acetaminophen.
- 3 or more alcoholic drinks every day while using this product
Allergy alert:
acetaminophen may cause severe skin or severe allergic reactions, Symptoms may include:
- skin reddening
- blisters
- rash
- hives
- facial swelling
- asthma (wheezing)
- shock
If a skin or general allergic reactions occurs, stop use and seek medical help right away
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen ask a doctor or pharmacist
- if you have ever had an allertgic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- liver disease
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
- a breathing problem such as emphysema or chronic bronchitis
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
When using this product
- you may get drowsy
- avoid alcoholic drinks
- excitability may occur, especially in children
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- limit the use of caffeine-containing medications, foods, or beverages because too much caffeine may cause nervousness, irritability sleeplessness, and, occasionally rapid heartbeat. The recommended dose of this product contains about as much caffeine as a cup of coffee.
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
-
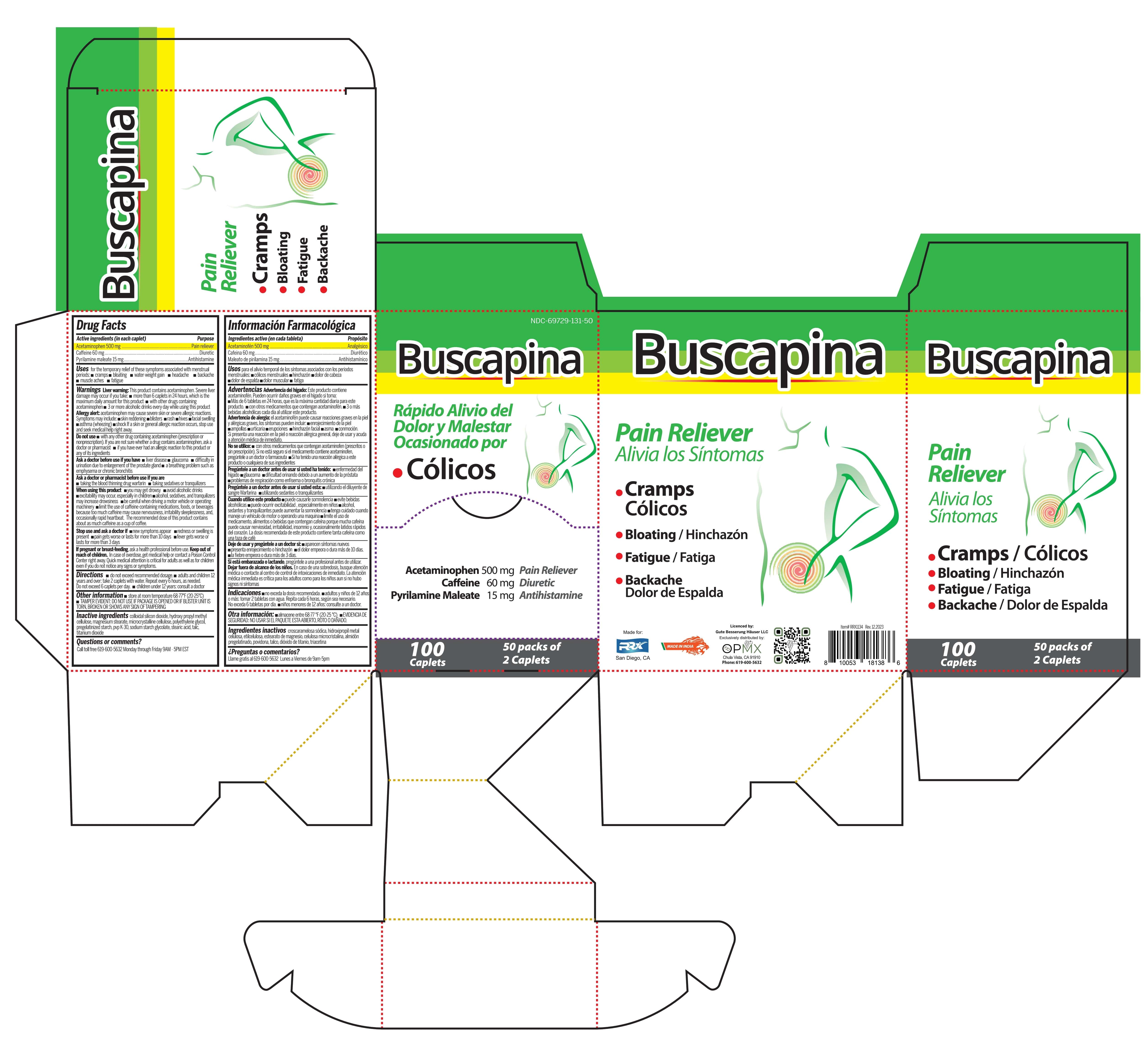
Principal Display Panel - Buscapina 144 Caplets
Buscapina
Acetaminophen, Caffeine, Pyrilamine Maleate Tablets
NDC 69729-131-72
Acetaminophen 500 mg . . . . . . . Pain reliever
Caffeine 60 mg . . . . . . . . . . . . . . Diuretic
Pyrilamine Maleate 15 mg . . . . . Antihistamine
- Cramps / Colicos
- Bloating / Hinchzon
- Fatigue / Fatiga
- Heacache
Dolor de Cabeza
- Backache
Dolor de Espalda
144 Caplets
72 packs of 2 Caplets
Made in India
Exclusively distributed by:
OPMX
San Diego, CA 92154
Phone: 619-600-5632


- Principal Display Panel - Buscapina 100 Caplets
-
INGREDIENTS AND APPEARANCE
BUSCAPINA
acetaminophen, caffeine, pyrilamine maleate tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69729-131 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 60 mg PYRILAMINE MALEATE (UNII: R35D29L3ZA) (PYRILAMINE - UNII:HPE317O9TL) PYRILAMINE MALEATE 15 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POVIDONE (UNII: FZ989GH94E) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) WATER (UNII: 059QF0KO0R) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white Score score with uneven pieces Shape CAPSULE Size 17mm Flavor Imprint Code V1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69729-131-72 72 in 1 CARTON 06/06/2022 1 2 in 1 PACKET; Type 0: Not a Combination Product 2 NDC:69729-131-50 50 in 1 CARTON 12/05/2023 2 2 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 06/06/2022 Labeler - OPMX LLC (029918743) Establishment Name Address ID/FEI Business Operations Vovantis Laboratories Private Limited 650502151 manufacture(69729-131)