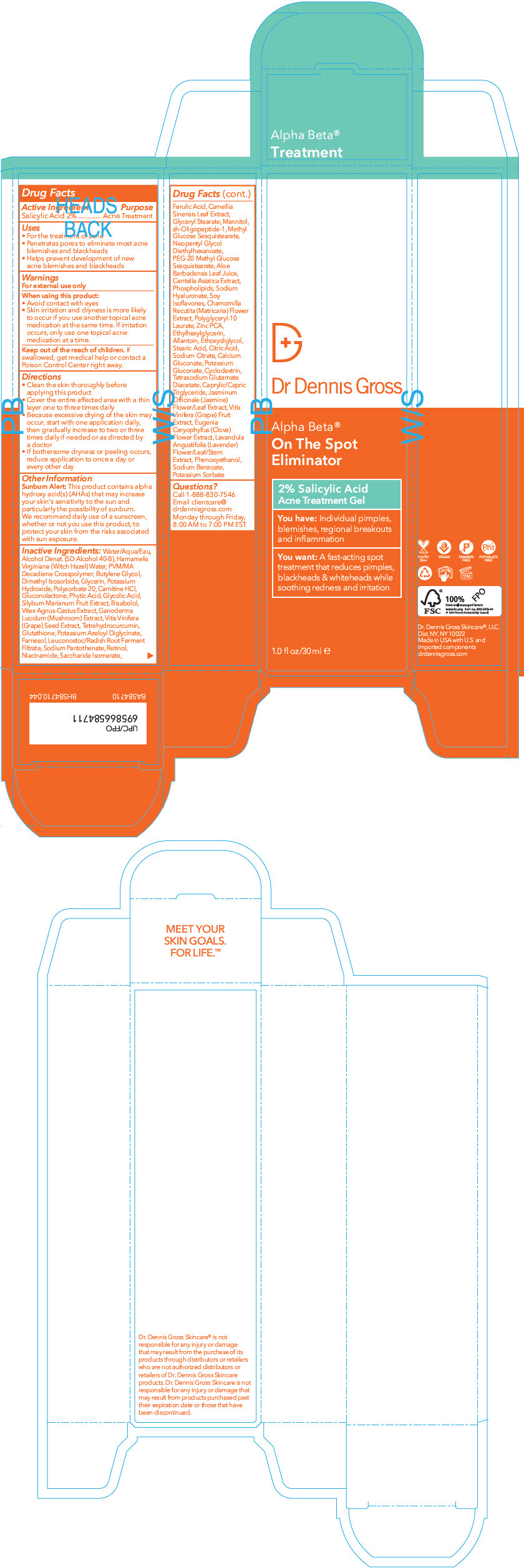
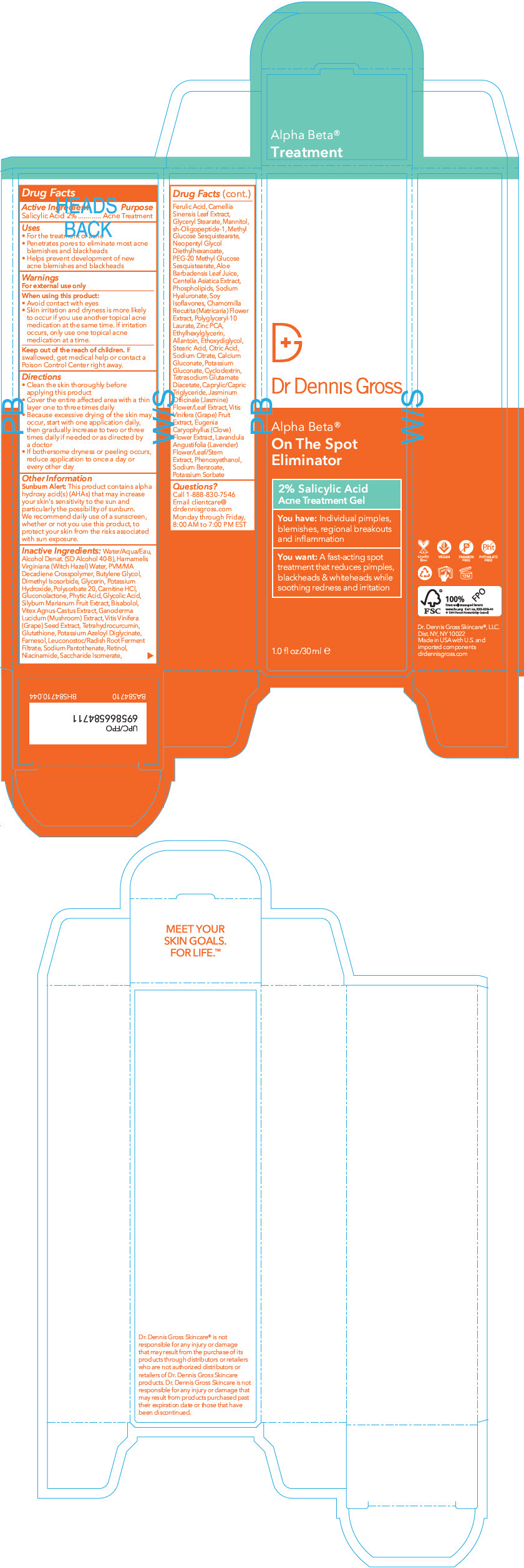
Label: DR. DENNIS GROSS ALPHA BETA ON THE SPOT ELIMINATOR- salicylic acid gel
- NDC Code(s): 51326-057-01
- Packager: Topiderm, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
- Warnings
-
Directions
- Clean the skin thoroughly before applying this product.
- Cover the entire affected area with a thin layer one to three times daily.
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- If bothersome dryness or peeling occur, reduce application to once a day or every other day
-
Other Information
Sunburn alert
This product contains alpha hydroxy acid(s) (AHAs) that may increase your skin's sensitivity to the sun and particularly the possibility of sunburn. We recommend daily use of a sunscreen, whether or not you use this product, to protect your skin from the risks associated with sun exposure.
-
Inactive ingredients
Water/Aqua/Eau, Alcohol Denat. (SD Alcohol 40-B), Hamamelis Virginiana (Witch Hazel) Water, PVM/MA Decadiene Crosspolymer, Butylene Glycol, Dimethyl Isosorbide, Glycerin, Potassium Hydroxide, Polysorbate 20, Carnitine HCl, Gluconolactone, Phytic Acid, Glycolic Acid, Silybum Marianum Fruit Extract, Bisabolol, Vitex Agnus-Castus Extract, Ganoderma Lucidum (Mushroom) Extract, Vitis Vinifera (Grape) Seed Extract, Tetrahydrocurcumin, Glutathione, Potassium Azeloyl Diglycinate, Farnesol, Leuconostoc/Radish Root Ferment Filtrate, Sodium Pantothenate, Retinol, Niacinamide, Saccharide Isomerate, Ferulic Acid, Camellia Sinensis Leaf Extract, Glyceryl Stearate, Mannitol, sh-Oligopeptide-1, Methyl Glucose Sesquistearate, Neopentyl Glycol Diethylhexanoate, PEG-20 Methyl Glucose Sesquistearate, Aloe Barbadensis Leaf Juice, Centella Asiatica Extract, Phospholipids, Sodium Hyaluronate, Soy Isoflavones, Chamomilla Recutita (Matricaria) Flower Extract, Polyglyceryl-10 Laurate, Zinc PCA, Ethylhexylglycerin, Allantoin, Ethoxydiglycol, Stearic Acid, Citric Acid, Sodium Citrate, Calcium Gluconate, Potassium Gluconate, Cyclodextrin, Tetrasodium Glutamate Diacetate, Caprylic/Capric Triglyceride, Jasminum Officinale (Jasmine) Flower/Leaf Extract, Vitis Vinifera (Grape) Fruit Extract, Eugenia Caryophyllus (Clove) Flower Extract, Lavandula Angustifolia (Lavender) Flower/Leaf/Stem Extract, Phenoxyethanol, Sodium Benzoate, Potassium Sorbate
- Questions?
- PRINCIPAL DISPLAY PANEL - 30 ml Tube Box
-
INGREDIENTS AND APPEARANCE
DR. DENNIS GROSS ALPHA BETA ON THE SPOT ELIMINATOR
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51326-057 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) HAMAMELIS VIRGINIANA LEAF WATER (UNII: 8FP93ED6H2) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) GLYCERIN (UNII: PDC6A3C0OX) POTASSIUM HYDROXYCITRATE (UNII: 4586270U1R) POLYSORBATE 20 (UNII: 7T1F30V5YH) CARNITINE HYDROCHLORIDE, (+)- (UNII: 11X758JML9) GLUCONOLACTONE (UNII: WQ29KQ9POT) FYTIC ACID (UNII: 7IGF0S7R8I) GLYCOLIC ACID (UNII: 0WT12SX38S) SILYBUM MARIANUM WHOLE (UNII: JLK089424F) .ALPHA.-BISABOLOL, (+)- (UNII: 105S6I733Z) VITEX AGNUS-CASTUS LEAF (UNII: W4U9Y3Q5SR) REISHI (UNII: TKD8LH0X2Z) TETRAHYDRODIFERULOYLMETHANE (UNII: 00U0645U03) GLUTATHIONE (UNII: GAN16C9B8O) POTASSIUM AZELOYL DIGLYCINATE (UNII: N02RVN6NYP) FARNESOL (UNII: EB41QIU6JL) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) SODIUM PANTOTHENATE (UNII: OES0R93F0C) RETINOL (UNII: G2SH0XKK91) NIACINAMIDE (UNII: 25X51I8RD4) SACCHARIDE ISOMERATE (UNII: W8K377W98I) FERULIC ACID (UNII: AVM951ZWST) GREEN TEA LEAF (UNII: W2ZU1RY8B0) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) MANNITOL (UNII: 3OWL53L36A) NEPIDERMIN (UNII: TZK30RF92W) METHYL GLUCOSE SESQUISTEARATE (UNII: V1YW10H14D) ALOE VERA LEAF (UNII: ZY81Z83H0X) CENTELLA ASIATICA LEAF (UNII: 6810070TYD) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GENISTEIN (UNII: DH2M523P0H) MATRICARIA CHAMOMILLA LEAF (UNII: 6I9LN466F0) POLYGLYCERYL-10 LAURATE (UNII: MPJ2Q8WI8G) ZINC PIDOLATE (UNII: C32PQ86DH4) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ALLANTOIN (UNII: 344S277G0Z) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) STEARIC ACID (UNII: 4ELV7Z65AP) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) CALCIUM GLUCONATE (UNII: SQE6VB453K) POTASSIUM GLUCONATE (UNII: 12H3K5QKN9) CYCLODEXTRINS (UNII: 7E6SK9QDT8) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) JASMINUM OFFICINALE FLOWER (UNII: 0Q8K841432) VITIS VINIFERA FRUIT OIL (UNII: YQ5Q4Y2Z8U) CLOVE LEAF OIL (UNII: VCA5491KVF) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51326-057-01 1 in 1 BOX 11/17/2023 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH DRUG M006 11/17/2023 Labeler - Topiderm, Inc (049121643) Registrant - Topiderm, Inc. (049121643) Establishment Name Address ID/FEI Business Operations Topiderm, Inc 049121643 MANUFACTURE(51326-057) Establishment Name Address ID/FEI Business Operations Topix Pharmaceuticals, Inc. 117745066 PACK(51326-057)