Label: PROPOFOL 1% injection, emulsion
-
NDC Code(s):
80700-113-10,
80700-113-11,
80700-113-12,
80700-113-20, view more80700-113-21, 80700-113-22
- Packager: Genixus
- This is a repackaged label.
- Source NDC Code(s): 43598-549
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Propofol injectable emulsion, USP is a sterile, nonpyrogenic white or almost white, homogeneous
emulsion, containing 10 mg/mL of propofol, USP suitable for intravenous administration. Propofol,
USP is chemically described as 2,6-diisopropylphenol and has a molecular weight of 178.27.
The structural formula is: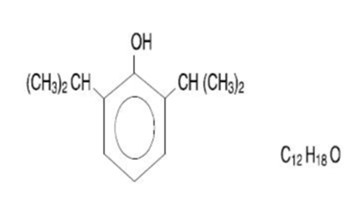
Propofol, USP is slightly soluble in water and, thus, is formulated in a white, oil-in-water emulsion.
The pKa is 11. The octanol/water partition coefficient for propofol, USP is 6761:1 at a pH of 4.5 to
7.4. In addition to the active component, propofol, USP the formulation also contains
soybean oil (100 mg/mL), glycerol (22.5 mg/mL), egg phospholipids (12 mg/mL); and benzyl alcohol
(0.15%); with sodium hydroxide to adjust pH. Propofol injectable emulsion, USP is isotonic and has
a pH of 5.5 to 7.4. - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PROPOFOL 1%
propofol 1% injection, emulsionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:80700-113(NDC:43598-549) Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PROPOFOL (UNII: YI7VU623SF) (PROPOFOL - UNII:YI7VU623SF) PROPOFOL 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) 22.5 mg in 1 mL SOYBEAN OIL (UNII: 241ATL177A) 100 mg in 1 mL EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) 12 mg in 1 mL BENZYL ALCOHOL (UNII: LKG8494WBH) 0.0015 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80700-113-11 10 in 1 BAG 03/29/2023 1 NDC:80700-113-10 10 mL in 1 SYRINGE, PLASTIC; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:80700-113-12 25 in 1 BAG 03/29/2023 2 NDC:80700-113-10 10 mL in 1 SYRINGE, PLASTIC; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 3 NDC:80700-113-21 10 in 1 BAG 03/29/2023 3 NDC:80700-113-20 20 mL in 1 SYRINGE, PLASTIC; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 4 NDC:80700-113-22 25 in 1 BAG 03/29/2023 4 NDC:80700-113-20 20 mL in 1 SYRINGE, PLASTIC; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/29/2023 Labeler - Genixus (117436254) Establishment Name Address ID/FEI Business Operations Genixus 117436254 repack(80700-113) , analysis(80700-113)




