Label: ASTEPRO ALLERGY- azelastine hcl spray, metered
-
NDC Code(s):
0280-0065-01,
0280-0065-02,
0280-0065-03,
0280-0065-04, view more0280-0065-05, 0280-0065-09, 0280-0065-10
- Packager: Bayer HealthCare LLC.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated September 21, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- Uses
-
WARNINGS
Warnings
Only for use in the nose. Do not spray in eyes or mouth.
Ask a doctor before use if you
- have had recent nose ulcers or nose surgery
- have had a nose injury that has not healed
When using this product
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- you may get a bitter taste in your mouth. To help avoid this, tilt your head downward while spraying.
- nasal discomfort or sneezing may occur right after use
- do not share this bottle with anyone else as this may spread germs
-
Directions
Read the User Guide for how to:
- prime the bottle before first use
- prime bottle again if not used for 3 or more days
- use the spray
- clean the spray nozzle if it gets clogged
adults and children 12 years and older This product may be used either once or twice a day:
- once daily: use 2 sprays in each nostril; OR
- twice daily: use 1 or 2 sprays in each nostril every 12 hours
- do not use more than 4 sprays in each nostril in a 24 hour period
children 6 years to 11 years
- an adult should supervise use
- 1 spray in each nostril every 12 hours
- do not use more than 2 sprays in each nostril in a 24 hour period
children under 6 years do not use - Other information
- INACTIVE INGREDIENT
- Questions or comments
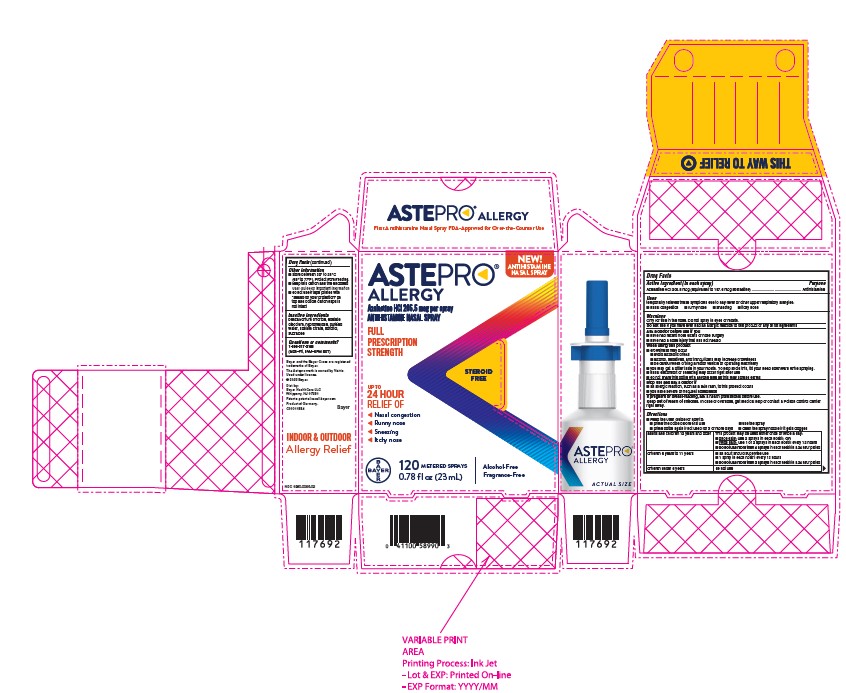
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ASTEPRO ALLERGY
azelastine hcl spray, meteredProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0280-0065 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AZELASTINE HYDROCHLORIDE (UNII: 0L591QR10I) (AZELASTINE - UNII:ZQI909440X) AZELASTINE HYDROCHLORIDE 205.5 ug Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) EDETATE DISODIUM (UNII: 7FLD91C86K) HYPROMELLOSES (UNII: 3NXW29V3WO) SODIUM CITRATE (UNII: 1Q73Q2JULR) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0280-0065-10 22 in 1 BOTTLE 07/05/2022 1 1 in 1 CARTON; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:0280-0065-03 120 in 1 BOTTLE 07/05/2022 2 3 in 1 PACKAGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 3 NDC:0280-0065-01 60 in 1 BOTTLE 07/05/2022 3 1 in 1 CARTON; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 4 NDC:0280-0065-02 120 in 1 BOTTLE 07/05/2022 4 1 in 1 CARTON; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 5 NDC:0280-0065-04 200 in 1 BOTTLE 07/05/2022 5 1 in 1 CARTON; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 6 NDC:0280-0065-09 120 in 1 BOTTLE 07/05/2022 6 2 in 1 CARTON; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 7 NDC:0280-0065-05 22 in 1 BOTTLE 01/24/2023 7 1 in 1 CARTON; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213872 06/27/2022 Labeler - Bayer HealthCare LLC. (112117283)