Label: BIMASONE- flumethasone injection, solution
- NDC Code(s): 61133-4009-1
- Packager: Bimeda, Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated April 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
BimasoneTM
(flumethasone) 0.5 mg/mL
STERILE INJECTION FOR HORSES, DOGS, & CATS
GlucocorticoidCAUTION
Federal law restricts this drug to use by or on the order of a licensed veterinarian.GENERAL
Flumethasone is a chemical modification of prednisolone which possesses greater anti-inflammatory and gluconeogenic properties than the parent compound when compared on an equivalent basis. Due to the potency of Bimasone, dosage recommendations should be consulted prior to drug administration. Chemically, it is 6α, 9α-difluoro-16α methylprednisolone.
The structural formula is as follows: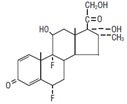
DESCRIPTION
The active ingredient of Bimasone is flumethasone which occurs as a white to creamy white, odorless,
crystalline powder. The appearance of Bimasone is a clear colorless to slightly yellowish mobile liquid.
Each mL of the injectable preparation contains 0.5 mg flumethasone, 420 mg polyethylene glycol 400, 9 mg benzyl alcohol as a preservative, 8 mg sodium chloride, 0.1 mg citric acid, sodium hydroxide and/or hydrochloric acid for pH adjustment when necessary, and water for injection USP, q.s. -
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY
Flumethasone has been reported (1) to possess 700 times the glucocorticoid activity of cortisol (hydrocortisone) as measured in the liver glycogen deposition assay in the rat; 120 times that of cortisol in the cotton pellet assay in the rat; and also in the same animal, shows a net excretion of sodium.
In similar tests in rats, another report (2) showed flumethasone 730 times more potent than cortisol in the granuloma inhibition assay and 165 times the activity of cortisol in the glycogen deposition assay. The same report showed that in man, the compound possessed 7.8 times the potency of prednisolone.
An additional report (3) indicated that flumethasone possessed 677 times the potency of cortisol in the liver glycogen deposition test in the rat, and 30, 25, and 31 times respectively, the eosinopenic, hyperglycemic and antirheumatic potency of cortisol, as measured in man.
Veterinary experimental studies utilizing the eosinophil depression test in normal dogs and blood glucose elevation and eosinophil depression in normal cattle as parameters of drug activity, in comparison tests involving prednisone and dexamethasone, indicate that flumethasone possesses greater anti-inflammatory and gluconeogenic activity than these compounds, on an equivalent basis.
Clinical evidence of drug potency obtained during evaluation of the compound and based upon effective
drug dosage levels further substantiates the above experimental findings.General Effects of Adrenocorticoids
The adrenocorticoids are divided into two main classes: mineralocorticoids and glucocorticoids, based on their major physiologic and pharmacologic actions.
Mineralocorticoids such as the naturally occurring desoxycorticosterone and aldosterone are mainly
concerned with hydration, sodium and potassium regulation and the normal renal glomerular filtration of these two electrolytes. The mineralocorticoids have little if any effect as anti-inflammatory agents, and are not widely used in medicine.
Glucocorticoids include the naturally occurring compounds, cortisone and hydrocortisone.
Their major effects are as follows:1. Increase protein catabolism and gluconeogenesis.
2. Depressionof lymphoid tissues, fibroblasts and eosinophils.
3. Increase the sense of well being and tolerance to pain.
4. Depress thyroid function and anterior pitutiary function through reciprocal influences.
5. Influence vasoconstrictive response of the circulatory system to norepinephrine, helping to maintain blood pressure.
6. Increase renal flow.
7. Influence gastric HCl and pepsin production.
8. Reduces the secretion of mucus from respiratory and enteric mucosa.
9. Affect to some degree sodium retention and potassium excretion.
10. Stimulate erythropoiesis and myelopoiesis.Synthetic analogues of cortisone and hydrocortisone containing a double bond between carbon 1 and 2 of the corticosteroid nucleus, resulted in compounds with a greatly decreased effect on electrolyte metabolism.
Additional molecular changes present in other synthetic corticoids presently used in medicine such as
methylation at carbons 6 or 16 and hydroxylation at carbon 16, have led to a further decrease in electrolyte imbalance noted with the naturally occurring glucocorticoids. Fluorination at carbon 6 and/or 9 have led to a marked increase in anti-inflammatory activity.
The synthetic glucocorticoids exhibit a marked increase in potency in that a smaller amount of drug is required to elicit the same effects seen only with large amounts of the natural glucocorticoids. It is also noted that the synthetic analogues persist for a longer period of time in the body which is believed due to their slower metabolism and excretion. -
INDICATIONS & USAGE
INDICATIONS
Bimasone is recommended for the various rheumatic, allergic, dermatologic and other disease states which are known to be responsive to the anti-inflammatory corticoids.
Equine Indications
1. Musculoskeletal conditions due to inflammation, where permanent structural changes do not exist,
such as bursitis, carpitis, osselets and myositis. Following therapy an appropriate period of rest should be instituted to allow a more normal return to function of the affected part.2. In allergic states such as hives, urticaria and insect bites.
Canine Indications
1. Musculoskeletal conditions due to inflammation of muscles or joints and accessory structures, where
permanent structural changes do not exist, such as arthritis, osteoarthritis, the disc syndrome and
myositis. In septic arthritis appropriate antibacterial therapy should be concurrently administered.2. In certain acute and chronic dermatoses of varying etiology to help control the pruritus, irritation and
inflammation associated with these conditions. The drug has proven useful in otitis externa in conjunction with topical medication for similar reasons.3. In allergic states such as hives, urticaria and insect bites.
4. Shock and shock-like states, (4) by intravenous administration.
Feline Indications
1. In certain acute and chronic dermatoses of varying etiology to help control the pruritus,
irritation and inflammation associated with these conditions. -
DOSAGE & ADMINISTRATION
ADMINISTRATION AND DOSAGE
Bimasone is recommended for administration by injection using various routes depending on the
animal species and conditions under treatment. Injection should be accomplished slowly, with the
drug at or near body temperature.The following recommended dosages should be used as therapeutic guides. Each animal should be treated on an individual basis and dosage adjusted according to the response noted.
Dosage of Bimasone:
Equine: 1.25 to 2.5 mg daily by intravenous, intramuscular or intra-articular injection. If
necessary, the dose may be repeated.Canine: 0.0625 to 0.25 mg daily by intravenous, intramuscular or subcutaneous injection. If
necessary, the dose may be repeated. With chronic conditions, the preceding doses may
be used and oral maintenance therapy with flumethasone tablets instituted at a daily
dose of 0.0625 to 0.25 mg.
lntralesional dosages in the dog have ranged from 0.125 to 1 mg depending on the size and
location of the lesion under treatment.
Intra-articular dosages in the dog have ranged from 0.166 to 1 mg depending on the severity of the condition under treatment and the size of the involved joint.
Feline: 0.03125 to 0.125 mg by intravenous, intramuscular or subcutaneous injection. If necessary, the dose may be repeated. With chronic conditions, the preceding injectable doses may be used and oral maintenance therapy with flumethasone tablets instituted at a daily dosage of 0.03125 to 0.125 mg.
NOTE: The use of a microsyringe or standard tuberculin syringe may facilitate the accurate
administration of small amounts of the drug.If desired, therapy with Bimasone may be substituted for other corticoids by the appropriate adjustment of dose levels.
-
PRECAUTIONS
PRECAUTIONS
The usual precautions and contraindications for adrenocorticoid hormones are applicable with this compound. The close observation of animals under treatment with this drug is necessary, since the usual signs of adrenocorticoid overdosage which include sodium retention, potassium loss, fluid retention, weight gain, etc., may not be readily observed.
Use of corticosteroids depending on dose duration and specific steroid may result in inhibition of endogenous steroid production following drug withdrawal. In patients presently receiving or recently withdrawn from systemic steroid treatments, therapy with a rapidly acting corticosteroid should be considered in unusually stressful situations.
Experimentally, it has been demonstrated that corticosteroids especially at high dose levels may result in delayed wound healing. An increase in the incidence of osteoporosis may be noted, mainly in the elderly, with the prolonged use of these compounds. Their use in older dogs and cats, during the healing stages of a bone fracture is not indicated for this reason. The intra-articular injection in leg injuries of the horse may produce osseous metaplasia.
If side effects occur, the veterinarian should be prepared to take the necessary steps to correct them, which consist of temporarily discontinuing therapy with the drug until the side effects disappear, when therapy may be resumed at a lower dose level.
When long-term therapy with corticosteroids is used, as is necessary on occasion in the dog and cat, the dose should be individually adjusted so that the minimum maintenance dose (which will keep the condition being treated under control) is desirable. In dogs and cats on long-term therapy with these drugs, a protein-rich diet is useful to counteract nitrogen loss if it should occur.
Similarly, a small amount of potassium chloride daily in the diet will counteract excessive potassium loss if this is present. Some natural dietary sources of potassium include dry nonfat milk solids, citrus juice, bran flakes, meat, fish and light cane molasses.
Bimasone may be administered to animals with bacterial diseases provided that specific and appropriate antibacterial therapy with antibiotic is administered simultaneously. In the absence of specific concomitant antimicrobial therapy, the prolonged use of corticosteroids is likely to lead to the spread of pathogenic microorganisms. The use of corticosteroids in such situations is not indicated. It should be borne in mind that flumethasone, like cortisone, through its anti-inflammatory action, may mask the usual signs of an infection such as pyrexia, inappetence, lassitude, etc. In the course of therapy with Bimasone, should the question of determining the presence of an infectious disease arise, the drug should be withheld temporarily until a diagnosis or rediagnosis establishes the facts.
SIDE EFFECTS
Side effects such as SAP and SGPT(ALT) enzyme elevations, weight loss, anorexia, polydipsia and polyuria
have occurred following parenteral or systemic use of synthetic corticosteroids. In dogs, vomiting and diarrhea, and Cushing's syndrome have been reported in association with prolonged or repeated steroid therapy.
Corticosteroids reportedly cause laminitis in horses. -
WARNINGS
WARNINGS
Clinical and experimental data have demonstrated that corticosteroids administered orally or by injection to animals may induce the first stage of parturition when administered during the last trimester of pregnancy and may precipitate premature parturition followed by dystocia, fetal death, retained placenta and metritis.
Additionally, corticosteroids administered to dogs, rabbits and rodents during pregnancy have resulted in cleft palate in offspring. Corticosteroids administered to dogs during pregnancy have also resulted in other congenital anomalies, including deformed forelegs, phocomelia and anasarca. - CONTRAINDICATIONS
-
SPL UNCLASSIFIED SECTION
CONTACT INFORMATION
To report suspected adverse drug events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Bimeda, Inc. at 1-888-524-6332. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae - HOW SUPPLIED
- STORAGE AND HANDLING
-
REFERENCES
REFERENCES
1. Fried, J.: “Biological Activities of Steroids in Relation to Cancer." Edited by G. Pincus and E. Volmer, Academic Press, New York and London, (1960), 9-24.
2. Boland, E.W.: Am. J. Med. 31, (Oct. 1961), 581-590.
3. Ringler, I., West, D., Dulin, W.E. and Boland, E.W.: Metabolism, Vol. 13, No. 1; (Jan. 1964), 37-44.
4. Lillehei, R.C., Longerbeam, J.K., Block, J.H. and Mannox, W.G.: Clin. Pharmacol. Ther. 5, (Jan.-Feb. 1964).Approved by FDA under ANADA # 200-612
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BIMASONE
flumethasone injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:61133-4009 Route of Administration INTRAVENOUS, INTRAMUSCULAR, INTRA-ARTICULAR, SUBCUTANEOUS, INTRALESIONAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUMETHASONE (UNII: LR3CD8SX89) (FLUMETHASONE - UNII:LR3CD8SX89) FLUMETHASONE .5 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) 420 mg in 1 mL benzyl alcohol (UNII: LKG8494WBH) 9 mg in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 8 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61133-4009-1 100 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200612 03/19/2024 Labeler - Bimeda, Inc. (060492923) Registrant - Bimeda, Inc. (060492923) Establishment Name Address ID/FEI Business Operations Bimeda-MTC Animal Health 256232216 manufacture


