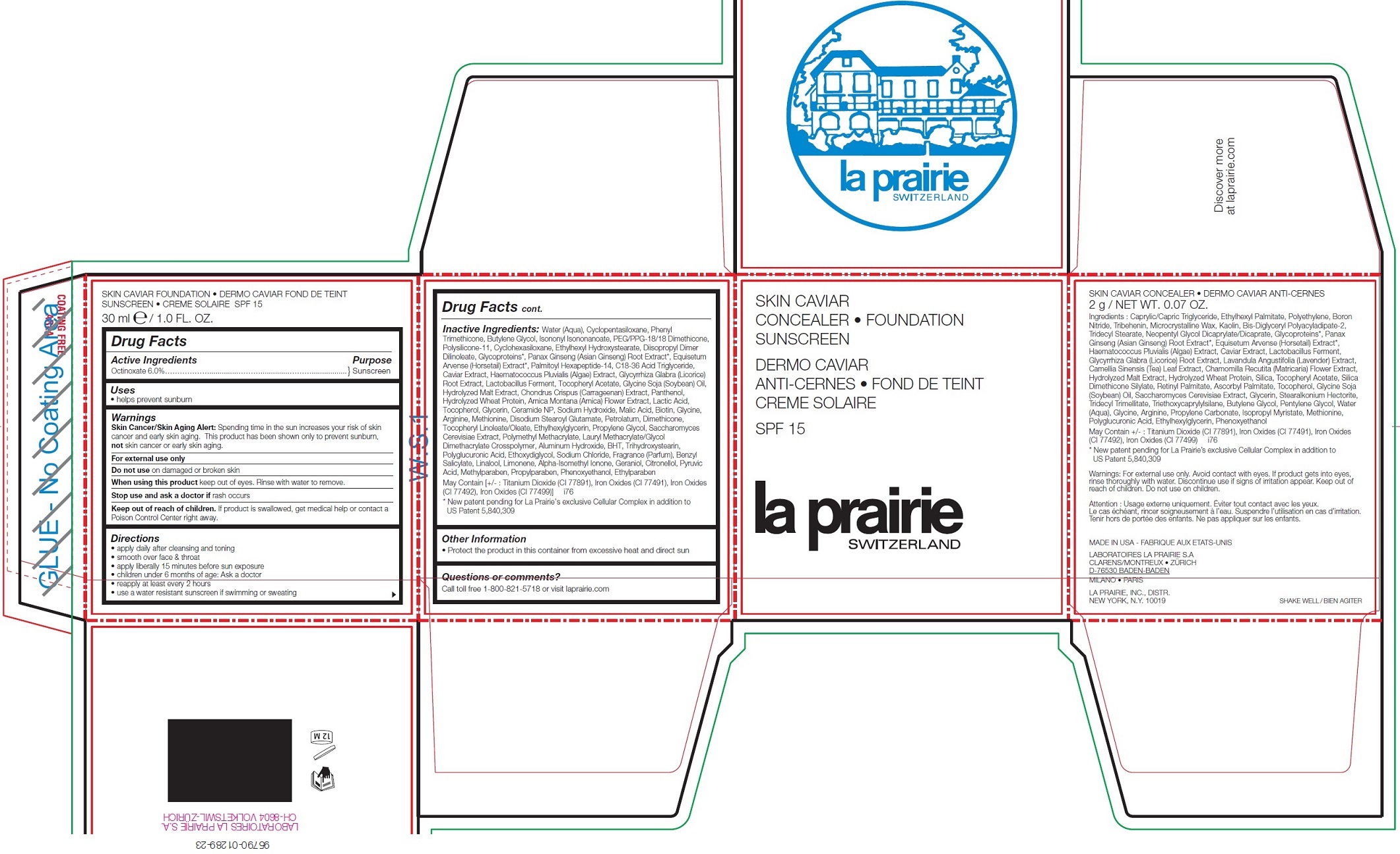
Label: LA PRAIRIE CONCEALER-FOUNDATION SUNSCREEN SPF 15 - ALMOND BEIGE- octinoxate kit
- NDC Code(s): 68026-816-30, 68026-817-30
- Packager: La Prairie, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Uses
- Warnings
- Directions
-
Inactive Ingredients:
Water (Aqua), Cyclopentasiloxane, Phenyl Trimethicone, Butylene Glycol, Isononyl Isononanoate, PEG/PPG-18/18 Dimethicone, Polysilicone-11, Cyclohexasiloxane, Ethylhexyl Hydroxystearate, Diisopropyl Dimer Dilinoleate, Glycoproteins*, Panax Ginseng (Asian Ginseng) Root Extract*, Equisetum Arvense (Horsetail) Extract*, Palmitoyl Hexapeptide-14, C18-36 Acid Triglyceride, Caviar Extract, Haematococcus Pluvialis (Algae) Extract, Glycyrrhiza Glabra (Licorice) Root Extract, Lactobacillus Ferment, Tocopheryl Acetate, Glycine Soja (Soybean) Oil, Hydrolyzed Malt Extract, Chondrus Crispus (Carrageenan) Extract, Panthenol, Hydrolyzed Wheat Protein, Arnica Montana (Arnica) Flower Extract, Lactic Acid, Tocopherol, Glycerin, Ceramide NP, Sodium Hydroxide, Malic Acid, Biotin, Glycine, Arginine, Methionine, Disodium Stearoyl Glutamate, Petrolatum, Dimethicone, Tocopheryl Linoleate/Oleate, Ethylhexylglycerin, Propylene Glycol, Saccharomyces Cerevisiae Extract, Polymethyl Methacrylate, Lauryl Methacrylate/Glycol Dimethacrylate Crosspolymer, Aluminum Hydroxide, BHT, Trihydroxystearin, Polyglucuronic Acid, Ethoxydiglycol, Sodium Chloride, Fragrance (Parfum), Benzyl Salicylate, Linalool, Limonene, Alpha-Isomethyl Ionone, Geraniol, Citronellol, Pyruvic Acid, Methylparaben, Propylparaben, Phenoxyethanol, Ethylparaben May Contain [+/- : Titanium Dioxide (CI 77891), Iron Oxides (CI 77491), Iron Oxides (CI 77492), Iron Oxides (CI 77499)] i76
* New patent pending for La Prairie’s exclusive Cellular Complex in addition to US Patent 5,840,309 - Other Information
- Questions or comments?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
LA PRAIRIE CONCEALER-FOUNDATION SUNSCREEN SPF 15 - ALMOND BEIGE
octinoxate kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68026-816 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68026-816-30 1 in 1 KIT 01/01/2014 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 30 mL Part 1 of 1 SKIN CAVIARCONCEALER-FOUNDATION SUNSCREEN SPF 15 ALMOND BEIGE
octinoxate creamProduct Information Item Code (Source) NDC:68026-817 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 60 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CYCLOMETHICONE 6 (UNII: XHK3U310BA) ETHYLHEXYL HYDROXYSTEARATE (UNII: B7I80BVV5E) DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) ASIAN GINSENG (UNII: CUQ3A77YXI) EQUISETUM ARVENSE BRANCH (UNII: 1L0VKZ185E) C18-36 ACID TRIGLYCERIDE (UNII: ZRA72DR3R7) CAVIAR, UNSPECIFIED (UNII: 020K6HLU0O) HAEMATOCOCCUS PLUVIALIS (UNII: 31T0FF0472) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SOYBEAN OIL (UNII: 241ATL177A) CHONDRUS CRISPUS CARRAGEENAN (UNII: UE856F2T78) PANTHENOL (UNII: WV9CM0O67Z) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) LACTIC ACID (UNII: 33X04XA5AT) TOCOPHEROL (UNII: R0ZB2556P8) GLYCERIN (UNII: PDC6A3C0OX) CERAMIDE NP (UNII: 4370DF050B) SODIUM HYDROXIDE (UNII: 55X04QC32I) MALIC ACID (UNII: 817L1N4CKP) BIOTIN (UNII: 6SO6U10H04) GLYCINE (UNII: TE7660XO1C) ARGININE (UNII: 94ZLA3W45F) METHIONINE (UNII: AE28F7PNPL) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) PETROLATUM (UNII: 4T6H12BN9U) DIMETHICONE (UNII: 92RU3N3Y1O) OLEIC ACID (UNII: 2UMI9U37CP) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SACCHAROMYCES CEREVISIAE (UNII: 978D8U419H) LAURYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EX0F4CZ66H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) TRIHYDROXYSTEARIN (UNII: 06YD7896S3) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) SODIUM CHLORIDE (UNII: 451W47IQ8X) BENZYL SALICYLATE (UNII: WAO5MNK9TU) LIMONENE, (+)- (UNII: GFD7C86Q1W) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) GERANIOL (UNII: L837108USY) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) PYRUVIC ACID (UNII: 8558G7RUTR) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLPARABEN (UNII: 14255EXE39) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68026-817-30 1 in 1 BOX 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/01/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/01/2014 Labeler - La Prairie, Inc. (606554996)