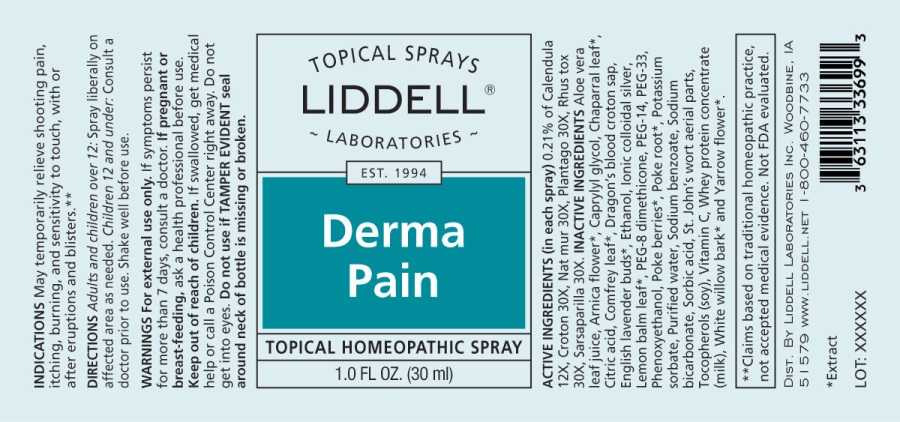
Label: DERMA PAIN (calendula officinalis, croton tiglium, natrum muriaticum, plantago major, rhus tox, sarsaparilla- smilax regelii spray
- NDC Code(s): 50845-0280-1
- Packager: Liddell Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated June 29, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS:
- INDICATIONS:
-
WARNINGS:
For external use only.
If symptoms persist for more than 7 days, consult a doctor.
If pregnant or breast feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or call a Poison Control Center right away.
Do not get into eyes
Do not use if TAMPER EVIDENT seal around neck of bottle is missing or broken.
- KEEP OUT OF REACH OF CHILDREN:
- DIRECTIONS:
- INDICATIONS:
-
INACTIVE INGREDIENTS:
Aloe Vera Leaf Juice, Arnica Flower*, Chaparral Leaf*, Citric Acid, Comfrey Leaf*, Dragon's Blood Croton (Sap), English Lavender (Buds)*, Ethanol, Ionic Colloidal Silver, Lemon Balm Leaf*, PEG-33, PEG-8 Dimethicone, PEG-14, Phenoxyethanol, Caprylyl Glycol, Sorbic Acid, Poke Berries*, Poke Root*, Potassium Sorbate, Purified Water, Sodium Bicarbonate, St. John's Wort Aerial Parts, Tocopherols (Vitamin E Oil), Vitamin C, Whey Protein Concentrate, White Willow Bark*, Yarrow Flower*.
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
DERMA PAIN
calendula officinalis, croton tiglium, natrum muriaticum, plantago major, rhus tox, sarsaparilla (smilax regelii) sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50845-0280 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 12 [hp_X] in 1 mL CROTON TIGLIUM SEED (UNII: 0HK2GZK66E) (CROTON TIGLIUM SEED - UNII:0HK2GZK66E) CROTON TIGLIUM SEED 30 [hp_X] in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 30 [hp_X] in 1 mL PLANTAGO MAJOR WHOLE (UNII: W2469WNO6U) (PLANTAGO MAJOR - UNII:W2469WNO6U) PLANTAGO MAJOR WHOLE 30 [hp_X] in 1 mL TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 30 [hp_X] in 1 mL SMILAX ORNATA ROOT (UNII: 2H1576D5WG) (SARSAPARILLA - UNII:2H1576D5WG) SMILAX ORNATA ROOT 30 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) LARREA TRIDENTATA LEAF (UNII: PK0TXD049P) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) COMFREY LEAF (UNII: DG4F8T839X) CROTON LECHLERI RESIN (UNII: GGG6W25C63) LAVANDULA ANGUSTIFOLIA SUBSP. ANGUSTIFOLIA FLOWER (UNII: 19AH1RAF4M) ALCOHOL (UNII: 3K9958V90M) SILVER (UNII: 3M4G523W1G) MELISSA OFFICINALIS LEAF (UNII: 50D2ZE9219) POLYETHYLENE GLYCOL 1600 (UNII: 1212Z7S33A) PEG-8 DIMETHICONE (UNII: GIA7T764OD) POLYETHYLENE GLYCOL 700 (UNII: 762678AC5R) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SORBIC ACID (UNII: X045WJ989B) PHYTOLACCA AMERICANA FRUIT (UNII: WE63661499) PHYTOLACCA AMERICANA ROOT (UNII: 11E6VI8VEG) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM BICARBONATE (UNII: 8MDF5V39QO) HYPERICUM PERFORATUM (UNII: XK4IUX8MNB) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) ASCORBIC ACID (UNII: PQ6CK8PD0R) WHEY (UNII: 8617Z5FMF6) SALIX ALBA BARK (UNII: 205MXS71H7) ACHILLEA MILLEFOLIUM FLOWER (UNII: YQR8R0SQEA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50845-0280-1 30 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 06/29/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/29/2022 Labeler - Liddell Laboratories, Inc. (832264241) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(50845-0280) , api manufacture(50845-0280) , label(50845-0280) , pack(50845-0280)