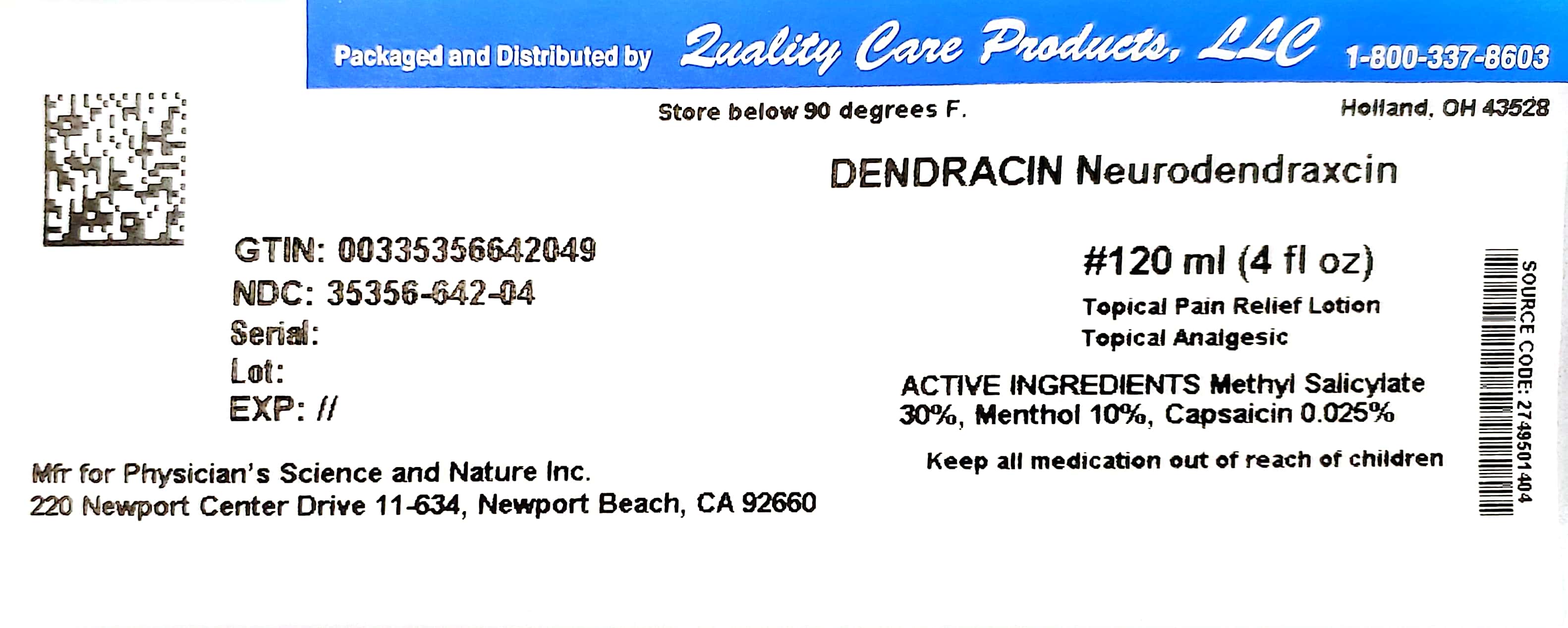
Label: DENDRACIN NEURODENDRAXCIN- methyl salicylate, menthol and capsaicin lotion
- NDC Code(s): 35356-642-04
- Packager: Quality Care Products, LLC
- This is a repackaged label.
- Source NDC Code(s): 27495-014
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses:
- Warnings:
- Keep away from children.
-
Directions:
Use only as directed. Shake before each use. Prior to first use, rub small amount to check for sensitivity. Gently rub over painful areas. Dry before contact with clothes or bedding to avoid staining. Wash hands after use. Do not use more than 4 times daily or if pregnant or nursing. If swallowed, call poison control. If placed into eyes, rinse with cold water and call a doctor.
- Do Not Use:
- Stop Use and Ask a Physician:
-
Inactive ingredients:
Water, benzocaine, glyceryl stearate, PEG 100 stearate, stearic acid, cetyl alcohol, propylene glycol, dimethyl sulfoxide, triethanolamine, poloxamer 407, aloe barbadensis gel, borage oil, ammonium acryloyldimethyltaurate, zingiber officinale root extract, methylparaben, propylparaben, soya lecithin, DMDM hydantoin, sodium stearoyl glutamate.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DENDRACIN NEURODENDRAXCIN
methyl salicylate, menthol and capsaicin lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:35356-642(NDC:27495-014) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 18 g in 60 mL MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 6 g in 60 mL CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.015 g in 60 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BENZOCAINE (UNII: U3RSY48JW5) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CETYL ALCOHOL (UNII: 936JST6JCN) DIMETHYL SULFOXIDE (UNII: YOW8V9698H) POLOXAMER 407 (UNII: TUF2IVW3M2) ALOE VERA LEAF (UNII: ZY81Z83H0X) BORAGE OIL (UNII: F8XAG1755S) AMMONIO METHACRYLATE COPOLYMER TYPE A (UNII: 8GQS4E66YY) GINGER (UNII: C5529G5JPQ) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) DMDM HYDANTOIN (UNII: BYR0546TOW) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:35356-642-04 120 mL in 1 BOTTLE; Type 1: Convenience Kit of Co-Package 11/14/2011 01/31/2026 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 11/14/2011 01/31/2026 Labeler - Quality Care Products, LLC (831276758) Establishment Name Address ID/FEI Business Operations Quality Care Products, LLC 831276758 relabel(35356-642)