Label: AVON ULTIMATE DAY MULTI-PERFORMANCE BROAD SPECTRUM SPF 25 SUNSCREEN- avobenzone, homosalate, octinoxate, oxybenzone cream
- NDC Code(s): 10096-0650-1
- Packager: The Avon Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
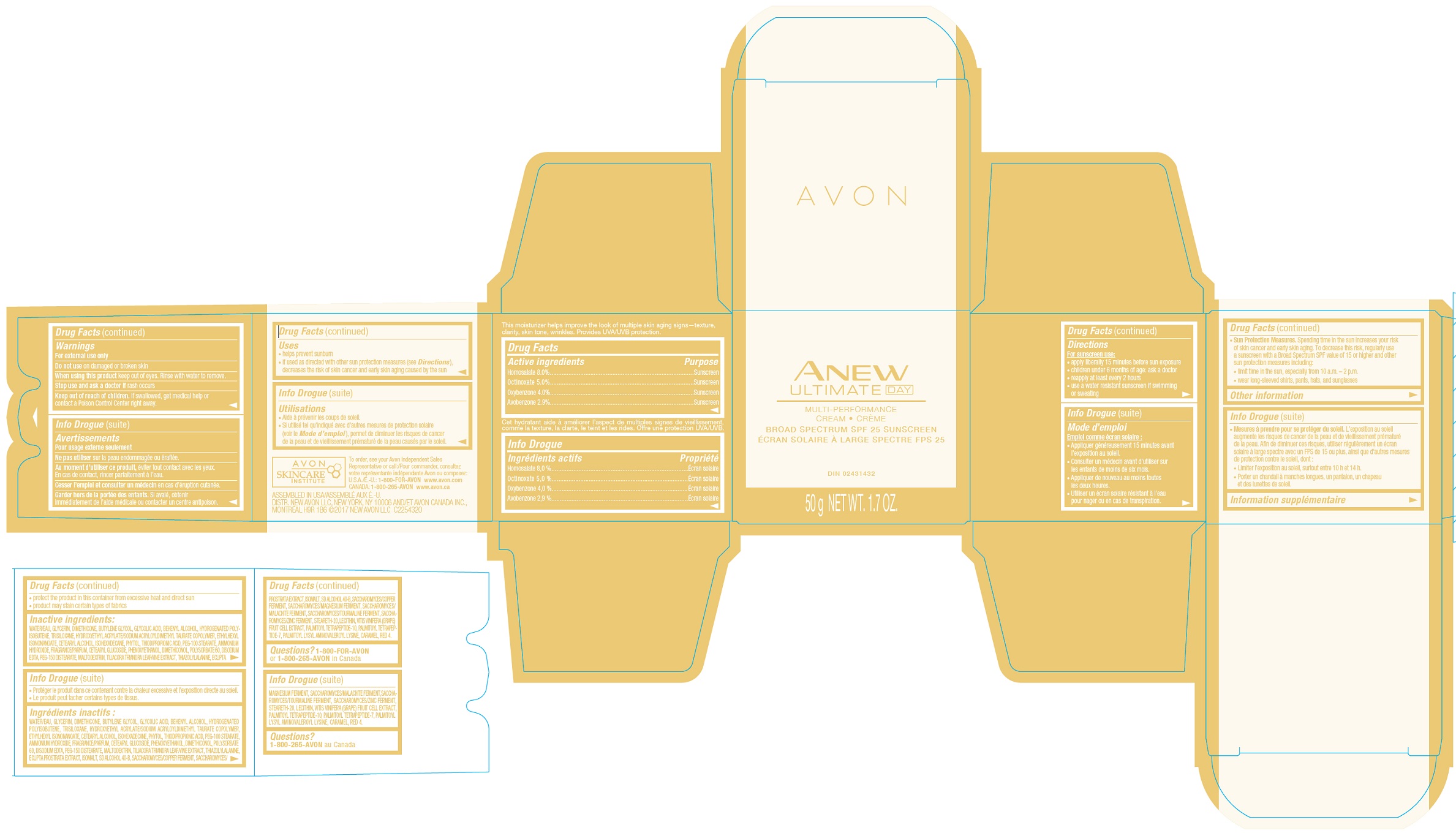
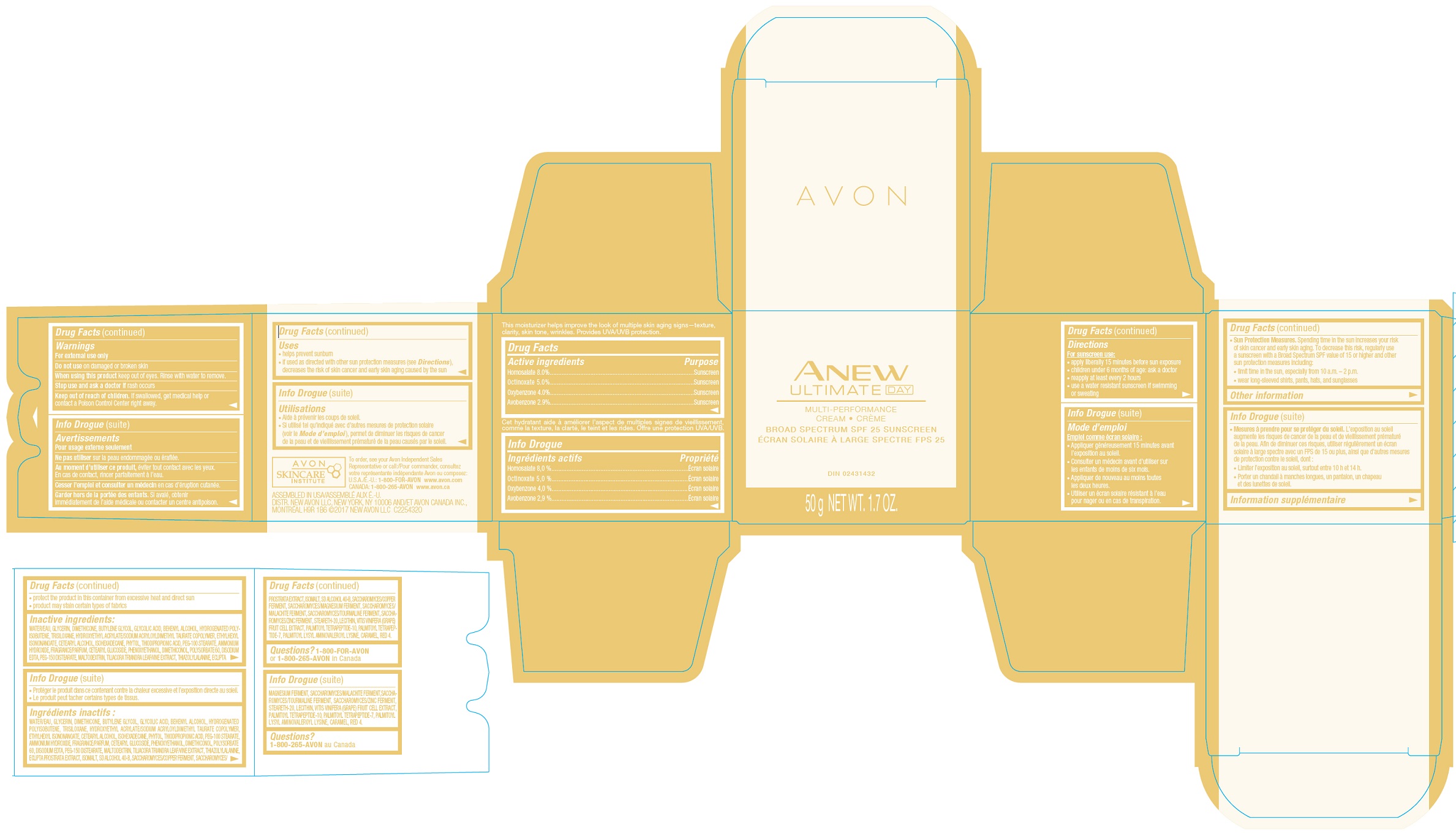
- Drug Facts
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
Directions
For sunscreen use:- apply liberally 15 minutes before sun exposure
- children less than 6 months of age: ask a doctor
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
Sun Protection Measures:
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
-
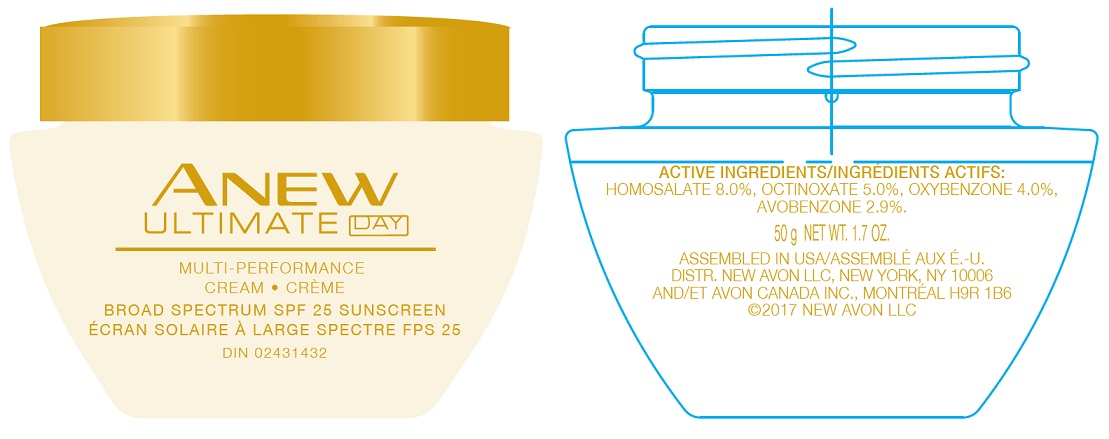
PRINCIPAL DISPLAY PANEL
AVON
ANEW
ULTIMATE DAYMULTI-PERFORMANCE
CREAM - CRÈME
BROAD SPECTRUM SPF 25 SUNSCREEN
ÉCRAN SOLAIRE À LARGE SPECTRE FPS 2550 g NET WT. 1.7 OZ.
U.S.A./E.-U.: 1-800-FOR-AVON
www.avon.com
CANADA: 1800-265-AVON
www.avon.caASSEMBLED IN USA/ASSEMBLÉ AUX E.-U.
DISTR. NEW AVON LLC, NEW YORK, NY 10006 AND/ET AVON CANADA, INC., MONTREAL H9R 1B6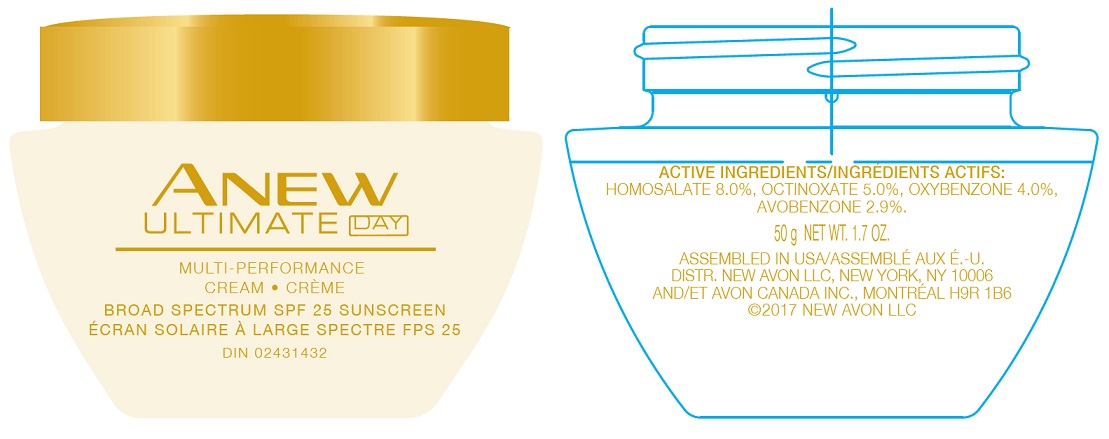
-
INGREDIENTS AND APPEARANCE
AVON ULTIMATE DAY MULTI-PERFORMANCE BROAD SPECTRUM SPF 25 SUNSCREEN
avobenzone, homosalate, octinoxate, oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10096-0650 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 5 mg in 1 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 8 mg in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 4 mg in 1 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2.9 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10096-0650-1 50 g in 1 JAR; Type 0: Not a Combination Product 05/27/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/27/2022 Labeler - The Avon Company (080143520)