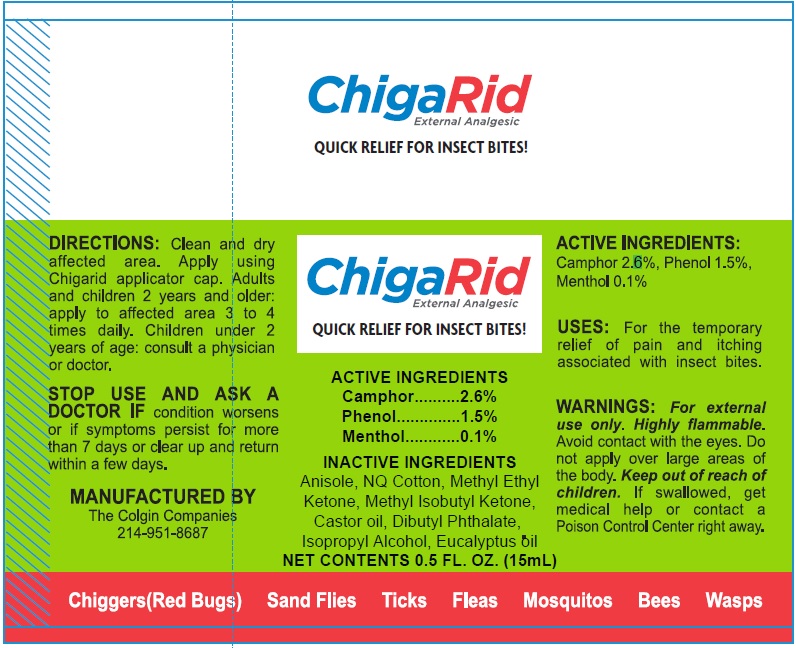
Label: CHIGARID (camphor- synthetic, phenol, menthol solution
- NDC Code(s): 47047-0001-1
- Packager: Colgin Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
- PURPOSE
- USES:
- WARNINGS:
- DIRECTIONS:
- INACTIVE INGREDIENTS
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
CHIGARID
camphor (synthetic), phenol, menthol solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:47047-0001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 26 mg in 1 mL PHENOL (UNII: 339NCG44TV) (PHENOL - UNII:339NCG44TV) PHENOL 15 mg in 1 mL MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength ANISOLE (UNII: B3W693GAZH) METHYL ETHYL KETONE (UNII: 6PT9KLV9IO) METHYL ISOBUTYL KETONE (UNII: U5T7B88CNP) CASTOR OIL (UNII: D5340Y2I9G) DIBUTYL PHTHALATE (UNII: 2286E5R2KE) ISOPROPYL ALCOHOL (UNII: ND2M416302) EUCALYPTUS OIL (UNII: 2R04ONI662) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47047-0001-1 1 in 1 CARTON 07/07/2011 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 07/07/2011 Labeler - Colgin Inc (799552443)