Label: GRANIX- tbo-filgrastim injection, solution
-
NDC Code(s):
63459-910-01,
63459-910-11,
63459-910-12,
63459-910-15, view more63459-910-17, 63459-910-18, 63459-910-36, 63459-912-01, 63459-912-11, 63459-912-12, 63459-912-15, 63459-912-17, 63459-912-18, 63459-912-36, 63459-918-53, 63459-918-59, 63459-920-53, 63459-920-59
- Packager: Cephalon, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated November 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use GRANIX safely and effectively. See full prescribing information for GRANIX.
GRANIX® (tbo-filgrastim) injection, for subcutaneous use
Initial U.S. Approval: 2012RECENT MAJOR CHANGES
Warnings and Precautions,
Myelodysplastic Syndrome and Acute Myeloid Leukemia
in Patients with Breast and Lung Cancer (5.8) 11/2023INDICATIONS AND USAGE
GRANIX (tbo-filgrastim) is a leukocyte growth factor indicated in adult and pediatric patients 1 month and older for reduction in the duration of severe neutropenia in patients with non-myeloid malignancies receiving myelosuppressive anticancer drugs associated with a clinically significant incidence of febrile neutropenia. (1)
DOSAGE AND ADMINISTRATION
-
Recommended dose: 5 mcg/kg per day administered as a subcutaneous injection
-
Administer the first dose no earlier than 24 hours following myelosuppressive chemotherapy. Do not administer within 24 hours prior to chemotherapy (2.1)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
Patients with a history of serious allergic reactions to filgrastim products or pegfilgrastim products. (4)
WARNINGS AND PRECAUTIONS
- Splenic Rupture: Evaluate patients who report left upper abdominal or shoulder pain for an enlarged spleen or splenic rupture (5.1)
- Acute Respiratory Distress Syndrome (ARDS): Monitor for and manage immediately. Discontinue GRANIX if suspected (5.2)
- Serious Allergic Reactions Including Anaphylaxis: Permanently discontinue GRANIX in patients with serious allergic reactions (5.3)
- Sickle Cell Disorders: Severe and sometimes fatal crisis can occur. Discontinue GRANIX if suspected (5.4)
- Glomerulonephritis: Evaluate and consider dose reduction or interruption of GRANIX if causality is likely (5.5)
- Capillary Leak Syndrome: Monitor if symptoms develop and administer standard symptomatic treatment (5.6)
- Myelodysplastic Syndrome (MDS) and Acute Myeloid Leukemia (AML): Monitor patients with breast and lung cancer using GRANIX in conjunction with chemotherapy and/or radiotherapy for signs and symptoms of MDS/AML. (5.8)
ADVERSE REACTIONS
- Most common adverse reaction (≥1%) to GRANIX is bone pain (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Teva Pharmaceuticals at 1‑866-832-8537 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
GRANIX should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. (8.1)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2023
-
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 General Considerations for Administration
2.3 Important Administration Instructions for Healthcare Professionals
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Splenic Rupture
5.2 Acute Respiratory Distress Syndrome
5.3 Serious Allergic Reactions
5.4 Sickle Cell Disorders
5.5 Glomerulonephritis
5.6 Capillary Leak Syndrome
5.7 Potential for Tumor Growth Stimulatory Effects on Malignant Cells
5.8 Myelodysplastic Syndrome and Acute Myeloid Leukemia in Patients with Breast and Lung Cancer
5.9 Leukocytosis
5.10 Simultaneous Use with Chemotherapy and Radiation Therapy Not Recommended
5.11 Nuclear Imaging
5.12 Aortitis
5.13 Alveolar Hemorrhage
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dose of GRANIX is 5 mcg/kg per day administered as a subcutaneous injection. Administer the first dose of GRANIX no earlier than 24 hours following myelosuppressive chemotherapy. Do not administer GRANIX within 24 hours prior to chemotherapy.
Daily dosing with GRANIX should continue until the expected neutrophil nadir is passed and the neutrophil count has recovered to the normal range. Monitor complete blood count (CBC) prior to chemotherapy and twice per week until recovery.
2.2 General Considerations for Administration
GRANIX may be administered by either a healthcare professional, a patient or caregiver. Before a decision is made to allow GRANIX to be administered by a patient or caregiver, ensure that the patient is an appropriate candidate for self-administration or administration by a caregiver. Proper training on storage, preparation, and administration technique should be provided. If a patient or caregiver is not an appropriate candidate for any reason, then in such patients, GRANIX should be administered by a healthcare professional.
Dispense only the prefilled syringe without a safety needle guard device to patient or caregiver. Instruct patients and caregivers to follow the Instructions for Use provided with the GRANIX prefilled syringe to properly administer an injection after training by a healthcare professional.
Visually inspect parenteral drug products for particulate matter and discoloration prior to administration. Do not administer GRANIX if discoloration or particulates are observed.
The prefilled syringe and vial are for single-dose only. Discard unused portions. GRANIX and all its components are not made with natural rubber latex.
Recommended sites for subcutaneous GRANIX injections include the abdomen (except for the two-inch area around the navel), the front of the middle thighs, the upper outer areas of the buttocks, or the upper back portion of the upper arms. The injection site should be varied daily. GRANIX should not be injected into an area that is tender, red, bruised, or hard, or that has scars or stretch marks.
2.3 Important Administration Instructions for Healthcare Professionals
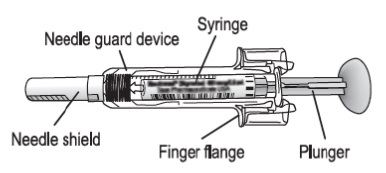
Hold the syringe assembly by the open sides of the device and remove the needle shield.
Expel any extra volume depending on dose needed.
Inject GRANIX subcutaneously as recommended [see Dosage and Administration (2.2)].
Push the plunger as far as it will go to inject all the medication. Injection of the entire prefilled syringe contents is necessary to activate the needle guard.
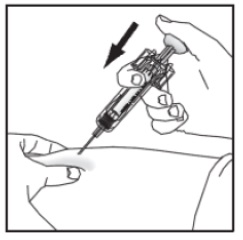
With the plunger still pressed all the way down, remove the needle from the skin.
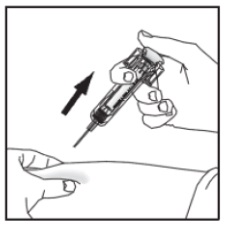
Slowly let go of the plunger and allow the empty syringe to move up inside the device until the entire needle is guarded.
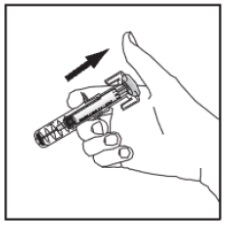
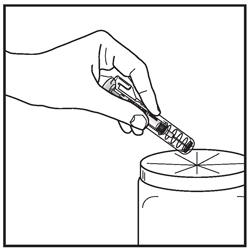
Discard the syringe assembly in approved containers.
-
3 DOSAGE FORMS AND STRENGTHS
GRANIX is a clear, colorless, preservative-free solution available as:
Prefilled Syringe:
Injection: 300 mcg/0.5 mL solution in single-dose prefilled syringe
Injection: 480 mcg/0.8 mL solution in single-dose prefilled syringe
Vial:
Injection: 300 mcg/mL solution in single-dose vial
Injection: 480 mcg/1.6 mL (300 mcg/mL) solution in single-dose vial
-
4 CONTRAINDICATIONS
GRANIX is contraindicated in patients with a history of serious allergic reactions to filgrastim products or pegfilgrastim products [see Warnings and Precautions (5.3)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Splenic Rupture
Splenic rupture, including fatal cases, can occur following administration of filgrastim products. Evaluate patients who report upper abdominal or shoulder pain for an enlarged spleen or splenic rupture. Discontinue GRANIX if splenic rupture is suspected or confirmed.
5.2 Acute Respiratory Distress Syndrome
Acute respiratory distress syndrome (ARDS) can occur in patients receiving filgrastim products. Evaluate patients who develop fever and lung infiltrates or respiratory distress after receiving GRANIX, for ARDS. Discontinue GRANIX in patients with ARDS.
5.3 Serious Allergic Reactions
Serious allergic reactions, including anaphylaxis, can occur in patients receiving GRANIX. Reactions can occur on initial exposure. The administration of antihistamines‚ steroids‚ bronchodilators‚ and/or epinephrine may reduce the severity of the reactions. Permanently discontinue GRANIX in patients with serious allergic reactions. Do not administer GRANIX to patients with a history of serious allergic reactions to filgrastim or pegfilgrastim.
5.4 Sickle Cell Disorders
Severe and sometimes fatal sickle cell crises can occur in patients with sickle cell disorders receiving filgrastim products. Discontinue GRANIX if sickle cell crisis occurs.
5.5 Glomerulonephritis
Glomerulonephritis can occur in patients receiving filgrastim products. The diagnoses were based on azotemia, hematuria (microscopic and macroscopic), proteinuria, and renal biopsy. Generally, events of glomerulonephritis resolved after dose reduction or discontinuation of the filgrastim product. If glomerulonephritis is suspected, evaluate for cause. If causality is likely, consider dose reduction or interruption of GRANIX.
5.6 Capillary Leak Syndrome
Capillary leak syndrome (CLS) can occur in patients receiving filgrastim products and is characterized by hypotension, hypoalbuminemia, edema and hemoconcentration. Episodes vary in frequency, severity and may be life-threatening if treatment is delayed. Patients who develop symptoms of capillary leak syndrome should be closely monitored and receive standard symptomatic treatment, which may include a need for intensive care.
5.7 Potential for Tumor Growth Stimulatory Effects on Malignant Cells
The granulocyte colony-stimulating factor (G‑CSF) receptor through which GRANIX acts has been found on tumor cell lines. The possibility that GRANIX acts as a growth factor for any tumor type, including myeloid malignancies and myelodysplasia, diseases for which GRANIX is not approved, cannot be excluded.
5.8 Myelodysplastic Syndrome and Acute Myeloid Leukemia in Patients with Breast and Lung Cancer
Patients with Severe Chronic Neutropenia
Confirm the diagnosis of SCN before initiating GRANIX therapy.
MDS and AML have been reported to occur in the natural history of congenital neutropenia without cytokine therapy. Cytogenetic abnormalities, transformation to MDS, and AML have also been observed in patients treated with GRANIX for SCN. Based on available data including a postmarketing surveillance study, the risk of developing MDS and AML appears to be confined to the subset of patients with congenital neutropenia. Abnormal cytogenetics and MDS have been associated with the eventual development of myeloid leukemia. The effect of GRANIX on the development of abnormal cytogenetics and the effect of continued GRANIX administration in patients with abnormal cytogenetics or MDS are unknown. Monitor patients for signs and symptoms of MDS/AML in these settings. If a patient with SCN develops abnormal cytogenetics or myelodysplasia‚ the risks and benefits of continuing GRANIX should be carefully considered.
Patients with Breast and Lung Cancer
MDS and AML have been associated with the use of GRANIX in conjunction with chemotherapy and/or radiotherapy in patients with breast and lung cancer. Monitor patients for signs and symptoms of MDS/AML in these settings.
5.9 Leukocytosis
White blood cell counts of 100‚000/mm3 or greater were observed in approximately 2% of patients receiving filgrastim products at dosages above 5 mcg/kg/day. In patients with cancer receiving GRANIX as an adjunct to myelosuppressive chemotherapy‚ to avoid the potential risks of excessive leukocytosis‚ it is recommended that GRANIX therapy be discontinued if the ANC surpasses 10‚000/mm3 after the chemotherapy-induced ANC nadir has occurred. Monitor CBCs at least twice weekly during therapy. Dosages of GRANIX that increase the ANC beyond 10‚000/mm3 may not result in any additional clinical benefit. In patients with cancer receiving myelosuppressive chemotherapy‚ discontinuation of filgrastim products therapy usually resulted in a 50% decrease in circulating neutrophils within 1 to 2 days‚ with a return to pretreatment levels in 1 to 7 days.
5.10 Simultaneous Use with Chemotherapy and Radiation Therapy Not Recommended
The safety and efficacy of filgrastim products, including GRANIX, given simultaneously with cytotoxic chemotherapy have not been established. Because of the potential sensitivity of rapidly dividing myeloid cells to cytotoxic chemotherapy‚ do not use GRANIX in the period 24 hours before through 24 hours after the administration of cytotoxic chemotherapy [see Dosage and Administration (2.2)].
The safety and efficacy of GRANIX have not been evaluated in patients receiving concurrent radiation therapy. Avoid the simultaneous use of GRANIX with chemotherapy and radiation therapy.
5.11 Nuclear Imaging
Increased hematopoietic activity of the bone marrow in response to growth factor therapy has been associated with transient positive bone-imaging changes. Consider this when interpreting bone-imaging results.
5.12 Aortitis
Aortitis has been reported in patients receiving another filgrastim product. It may occur as early as the first week after start of therapy. Manifestations may include generalized signs and symptoms such as fever, abdominal pain, malaise, back pain, and increased inflammatory markers (e.g., c‑reactive protein and white blood cell count). Consider aortitis in patients who develop these signs and symptoms without known etiology. Discontinue GRANIX if aortitis is suspected.
5.13 Alveolar Hemorrhage
Alveolar hemorrhage manifesting as pulmonary infiltrates and hemoptysis requiring hospitalization has been reported in healthy donors undergoing peripheral blood progenitor cell (PBPC) collection treated with another filgrastim product. Hemoptysis resolved with discontinuation of filgrastim. The use of GRANIX for PBPC mobilization in healthy donors is not an approved indication.
-
6 ADVERSE REACTIONS
The following potential serious adverse reactions are discussed in greater detail in other sections of the labeling:
- Splenic Rupture [see Warnings and Precautions (5.1)]
- Acute Respiratory Distress Syndrome [see Warnings and Precautions (5.2)]
- Serious Allergic Reactions [see Warnings and Precautions (5.3)]
- Sickle Cell Disorders [see Warnings and Precautions (5.4)]
- Glomerulonephritis [see Warnings and Precautions (5.5)]
- Capillary Leak Syndrome [see Warnings and Precautions (5.6)]
- Potential for Tumor Growth Stimulatory Effects on Malignant Cells [see Warnings and Precautions (5.7)]
- Myelodysplastic Syndrome and Acute Myeloid Leukemia [see Warnings and Precautions (5.8)]
- Leukocytosis [see Warnings and Precautions (5.9)]
- Simultaneous Use with Chemotherapy and Radiation Therapy Not Recommended [see Warnings and Precautions (5.10)]
- Aortitis [see Warnings and Precautions (5.12)]
- Alveolar Hemorrhage [see Warnings and Precautions (5.13)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Adverse Reactions in Adult Patients
GRANIX clinical trials safety data are based upon the results of three randomized clinical trials in patients receiving myeloablative chemotherapy for breast cancer (N=348), lung cancer (N=240) and non-Hodgkin’s lymphoma (N=92). In the breast cancer study, 99% of patients were female, the median age was 50 years, and 86% of patients were Caucasian. In the lung cancer study, 80% of patients were male, the median age was 58 years, and 95% of patients were Caucasian. In the non-Hodgkin’s lymphoma study, 52% of patients were male, the median age was 55 years, and 88% of patients were Caucasian. In all three studies a placebo (Cycle 1 of the breast cancer study only) or a non-U.S.-approved filgrastim product were used as controls. Both GRANIX and the non-U.S.-approved filgrastim product were administered at 5 mcg/kg subcutaneously once daily beginning one day after chemotherapy for at least five days and continued to a maximum of 14 days or until an ANC of ≥10,000 x 106/L after nadir was reached.
Bone pain was the most frequent treatment-emergent adverse reaction that occurred in at least 1% or greater in patients treated with GRANIX at the recommended dose and was numerically two times more frequent than in the placebo group. The overall incidence of bone pain in Cycle 1 of treatment was 3.4% (3.4% GRANIX, 1.4% placebo, 7.5% non-U.S.-approved filgrastim product).
Leukocytosis
In clinical studies, leukocytosis (WBC counts > 100,000 x 106/L) was observed in less than 1% patients with non-myeloid malignancies receiving GRANIX. No complications attributable to leukocytosis were reported in clinical studies.Additional Adverse Reactions
Other adverse reactions known to occur following administration of filgrastim products include myalgia, headache, vomiting, cutaneous vasculitis and thrombocytopenia.Adverse Reactions in Pediatric Patients
GRANIX clinical trials safety data in pediatric patients are based upon the results of one single-arm clinical trial in 50 pediatric patients who received myelosuppressive chemotherapy for treatment of solid tumors without marrow involvement [see Use in Special Populations (8.4)]. In this study, GRANIX was administered at 5 mcg/kg subcutaneously once daily beginning one day after chemotherapy. The most common (>5%) adverse reactions included thrombocytopenia (34%), pyrexia (8%), pain in extremity (6%), headache (6%) and diarrhea (6%).6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of GRANIX. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Sweet’s syndrome (acute febrile neutrophilic dermatosis), asthenia, diarrhea, and fatigue
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The limited published data on filgrastim product use during pregnancy are insufficient to inform a drug-associated risk. In animal reproduction studies, administration of tbo-filgrastim to pregnant rabbits during organogenesis resulted in increased spontaneous abortion and fetal malformations at systemic exposures 50 to 90 times the human exposure expected at the recommended human dose (see Data). GRANIX should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population(s) are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In an embryofetal developmental study, pregnant rabbits were administered subcutaneous doses of tbo-filgrastim during the period of organogenesis at 1, 10 and 100 mcg/kg/day. Increased abortions were evident in rabbits treated with tbo-filgrastim at 100 mcg/kg/day. This dose was maternally toxic as demonstrated by reduced body weight. Other embryofetal findings at this dose level consisted of post-implantation loss‚ decrease in mean live litter size and fetal weight, and fetal malformations such as malformed hind limbs and cleft palate. The dose of 100 mcg/kg/day corresponds to a systemic exposure (AUC) of approximately 50 to 90 times the exposures observed in patients treated with the clinical tbo-filgrastim dose of 5 mcg/kg/day.
8.2 Lactation
No data are available regarding the presence of tbo-filgrastim in human milk, the effects of the drug on the breastfed child, or the effects of the drug on milk production. Another filgrastim product was detected in human milk for up to 3 days after filgrastim administration. Because of the potential for serious adverse reactions in the breastfed child, including splenic rupture, acute respiratory distress syndrome and serious allergic reactions, advise patients not to breastfeed during treatment with GRANIX and for 2 weeks after the last dose.
8.4 Pediatric Use
The safety and effectiveness of GRANIX have been established for pediatric patients 1 month to < 17 years old (no data for the age group < 1 month old). Use of GRANIX in these age groups is supported by evidence from adequate and well-controlled studies of GRANIX in adults [see Clinical Studies (14)] with additional safety and pharmacokinetics data from a single-arm trial of 50 pediatric patients with solid tumors treated with GRANIX for chemotherapy-induced neutropenia. The 50 pediatric patients had a median age of 9.2 years (range, 1.4 to 15.9 years); 2 were infants (1 month to < 2 years old), 30 were children (2 to < 12 years old), and 18 were adolescents (12 to < 17 years old). The pharmacokinetics and safety profile of GRANIX in the pediatric population were similar to those seen in adults [see Adverse Reactions (6.1), Clinical Pharmacology (12.3)].
-
11 DESCRIPTION
GRANIX (tbo-filgrastim) is a non-glycosylated recombinant methionyl human granulocyte colony-stimulating growth factor (r-metHuG-CSF) manufactured by recombinant DNA technology using the bacterium strain E coli K802. It has a molecular weight of approximately 18.8 kDa and is composed of 175 amino acids. The endogenous human G-CSF is glycosylated and does not have the additional methionine amino acid residue in its NH2 terminal end.
The product is a sterile, clear, colorless, preservative-free solution containing tbo-filgrastim, glacial acetic acid, sorbitol, polysorbate 80, sodium hydroxide, and Water for Injection. The product is available in single-dose prefilled syringes that contain either 300 mcg or 480 mcg of tbo-filgrastim at a fill volume of 0.5 mL or 0.8 mL, respectively and single-dose vials that contain either 300 mcg or 480 mcg of tbo-filgrastim at a fill volume of 1 mL or 1.6 mL, respectively. See table below for product composition of each presentation.
Product Composition
300 mcg/0.5 mL
Syringe480 mcg/0.8 mL
Syringe300 mcg/1 mL
Vial480 mcg/1.6 mL
VialTbo-filgrastim 300 mcg
480 mcg 300 mcg 480 mcg Glacial Acetic Acid
0.3 mg 0.48 mg 0.6 mg 0.96 mg Polysorbate 80
0.0275 mg
0.044 mg
0.055 mg 0.088 mg Sorbitol 25 mg 40 mg
50 mg 80 mg Sodium Hydroxide q.s. to pH 4.2
q.s. to pH 4.2
q.s. to pH 4.2 q.s. to pH 4.2 Water for Injection q.s. to 0.5 mL
q.s. to 0.8 mL q.s. to 1 mL q.s. to 1.6 mL q.s. = quantity sufficient to make -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Tbo-filgrastim is a human granulocyte colony-stimulating factor (G-CSF) produced by recombinant DNA technology. Tbo-filgrastim binds to G-CSF receptors and stimulates proliferation of neutrophils. G-CSF is known to stimulate differentiation commitment and some end-cell functional activation, which increases neutrophil counts and activity.
12.2 Pharmacodynamics
The time to the maximum ANC level was between 3 to 5 days and returned to baseline by 21 days following completion of chemotherapy. Doubling the tbo-filgrastim subcutaneous dose from 5 mcg/kg to 10 mcg/kg resulted in a 16% to 19% increase in the maximum ANC level and a 33% to 36% increase in the area under the effect curve for ANC.
Cardiac Electrophysiology
At an intravenous dose of 5 mcg/kg, tbo-filgrastim did not prolong the QT interval to any clinically relevant extent.
12.3 Pharmacokinetics
Tbo-filgrastim exhibits nonlinear pharmacokinetics. Increasing the dose of subcutaneous GRANIX from 5 to 10 mcg/kg resulted in an approximate 2.5-fold increase in the maximum serum concentration (Cmax) and 3.0-fold increase in the area under the curve (AUC). In adult patients enrolled across three studies, subcutaneous GRANIX 5 mcg/kg resulted in median time to maximal serum tbo-filgrastim concentrations (Tmax) within 4 to 6 hours. Geometric mean [coefficient of variation (CV%)] serum Cmax was 20 to 31 ng/mL [24% to 65%] within 4 to 6 hours. Geometric mean serum tbo-filgrastim area under the curve (AUC0-12h) ranged from 151 to 227 ng/mL*h [24% to 60%]. No accumulation in serum tbo-filgrastim concentrations was observed after multiple dosing.
Absorption
The absolute bioavailability of 5 mcg/kg subcutaneous tbo-filgrastim was 33%.
Metabolism/Elimination
Tbo-filgrastim clearance is primarily dependent on G-CSF receptor-mediated clearance which can be saturated by high serum concentrations of tbo-filgrastim and diminished in neutropenia. The median serum elimination half-life of tbo-filgrastim (5 mcg/kg sc) was 3.0 to 3.5 hours.
Specific Populations
No sex-related differences were observed.
Pediatric Patients:
The geometric mean [coefficient of variation (CV%)] of Cmax was 18 ng/mL (56%) and AUC0-12h was 130 ng*hr/mL (52%) following subcutaneous administration of GRANIX 5 mcg/kg in 49 pediatric patients (1.4 to 15.9 years) after chemotherapy. No clinically relevant differences in the pharmacokinetics of GRANIX were observed between infants, children and adolescents.
Patients with Renal or Hepatic Impairment:
Mild renal impairment (creatinine clearance 60 to 89 mL/min by Cockcroft-Gault) had no effect on tbo-filgrastim pharmacokinetics. The pharmacokinetics in patients with moderate and severe renal impairment has not been studied. The pharmacokinetics in patients with hepatic impairment has not been studied.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of GRANIX or of other filgrastim products.
While available data suggest that 1.4% of patients developed binding antibodies to filgrastim, the nature and specificity of these antibodies has not been adequately studied. Because of the low occurrence of anti- drug antibodies, the effect of these antibodies on the pharmacokinetics, pharmacodynamics, safety, and/or effectiveness of GRANIX is unknown.
Cytopenias resulting from an antibody response to exogenous growth factors have been reported on rare occasions in patients treated with other recombinant growth factors.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity and genetic toxicology studies have not been conducted with tbo-filgrastim.
A fertility study was not conducted with tbo-filgrastim. Toxicology studies of up to 26 weeks in rats or monkeys did not reveal findings in male or female reproductive organs that would suggest impairment of fertility.
-
14 CLINICAL STUDIES
The efficacy of GRANIX was evaluated in a multinational, multicenter, randomized and controlled Phase 3 study in 348 chemotherapy-naive patients with high-risk stage II, stage III, or stage IV breast cancer receiving doxorubicin (60 mg/m2) and docetaxel (75 mg/m2) comparing GRANIX to placebo and a non-U.S.-approved filgrastim product as controls. The median age of the patients was 50 years (range 25 to 75 years) with 99% female and 86% Caucasian.
GRANIX, placebo, and the non-U.S.-approved filgrastim product were administered at 5 mcg/kg subcutaneously once daily beginning one day after chemotherapy for at least five days and continued to a maximum of 14 days or until an ANC of ≥10,000 x 106/L after nadir was reached.
GRANIX was superior to placebo in duration of severe neutropenia (DSN) with a statistically significant reduction in DSN (1.1 days vs. 3.8 days, p < 0.0001).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
GRANIX solution for injection is supplied as a single-dose, preservative-free clear solution, in either a vial or, a prefilled syringe made from Type I glass which has a permanently attached stainless steel needle. The active substance is tbo-filgrastim.
Prefilled Syringes (UltraSafe Passive® Needle Guard)
GRANIX 300 mcg/0.5 mL: Each prefilled syringe contains 300 mcg of tbo-filgrastim in 0.5 mL solution with a blue plunger in:
- Pack of 1 with a safety needle guard in blister: NDC 63459-910-11
- Packs of 10 with a safety needle guard in blisters: NDC 63459-910-15
GRANIX 480 mcg/0.8 mL: Each prefilled syringe contains 480 mcg of tbo-filgrastim in 0.8 mL solution with a clear plunger in:
- Pack of 1 with a safety needle guard in blister: NDC 63459-912-11
- Packs of 10 with a safety needle guard in blisters: NDC 63459-912-15
Prefilled Syringes
GRANIX 300 mcg/0.5 mL: Each prefilled syringe contains 300 mcg of tbo-filgrastim in 0.5 mL solution with a blue plunger in:
- Pack of 1 without a safety needle guard (for patients and caregivers): NDC 63459-910-17
- Packs of 5 without a safety needle guard (for patients and caregivers): NDC 63459-910-36
GRANIX 480 mcg/0.8 mL: Each prefilled syringe contains 480 mcg of tbo-filgrastim in 0.8 mL solution with a clear plunger in:
- Pack of 1 without a safety needle guard (for patients and caregivers): NDC 63459-912-17
- Packs of 5 without a safety needle guard (for patients and caregivers): NDC 63459-912-36
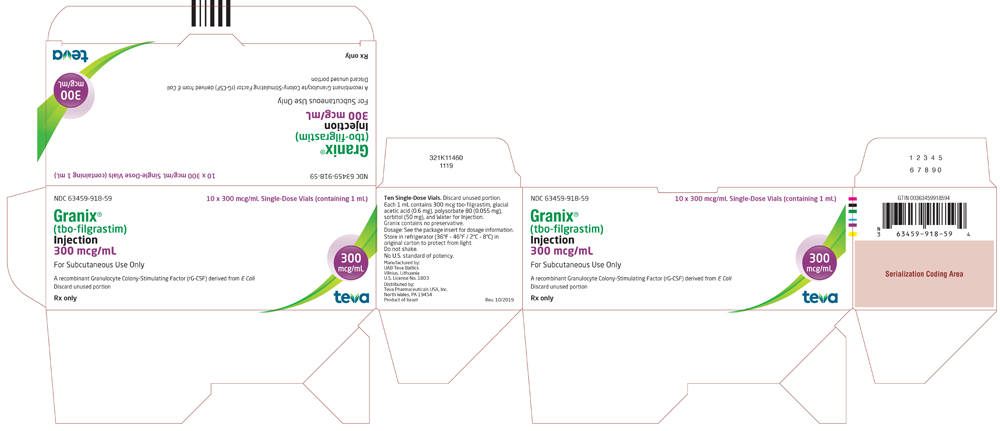
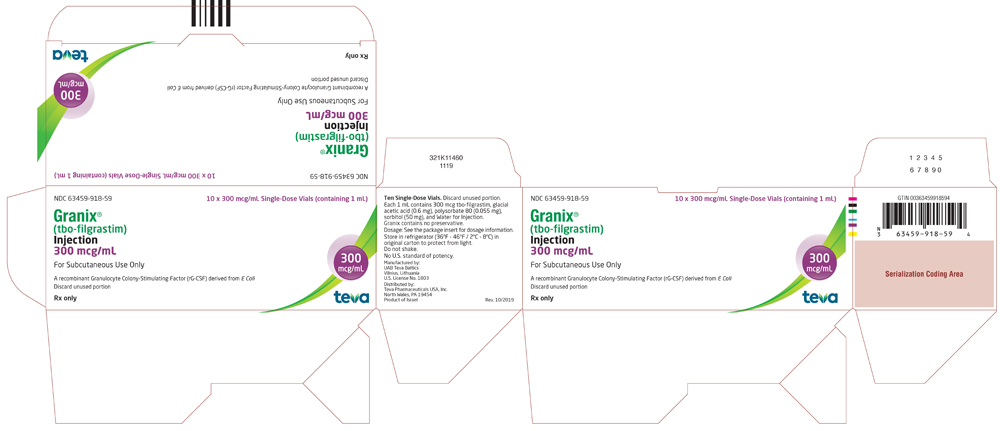
Vials
GRANIX 300 mcg/mL: Each vial contains 300 mcg of tbo-filgrastim in 1 mL solution.
- Packs of 10 single-dose vials: (NDC 63459-918-59)
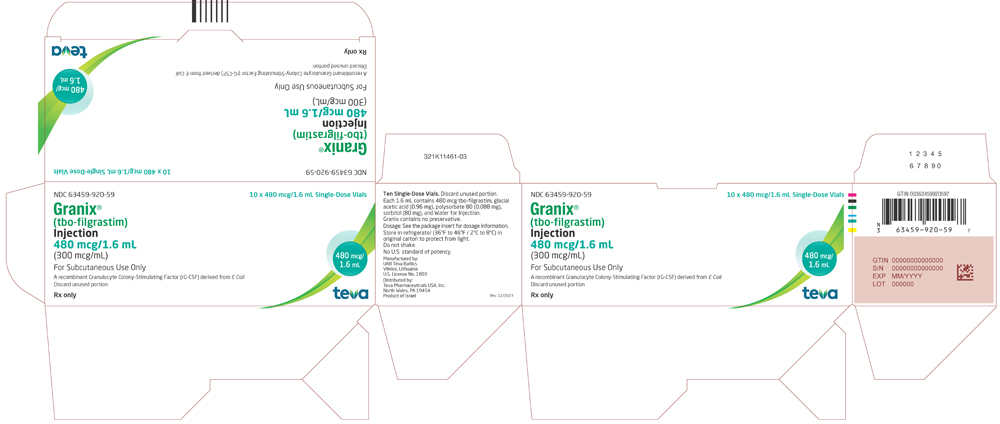
GRANIX 480 mcg/1.6 mL (300 mcg/mL): Each vial contains 480 mcg of tbo-filgrastim in 1.6 mL solution.
- Packs of 10 single-dose vials: (NDC 63459-920-59)
GRANIX and all its components are not made with natural rubber latex [see Dosage and Administration (2.2)].
Store GRANIX in a refrigerator at 36°F to 46°F (2°C to 8°C). Protect from light. Within its shelf life, the product may be removed from 36°F to 46°F (2°C to 8°C) storage for a single period of up to 5 days between 73°F to 81°F (23°C to 27°C). If not used within 5 days, the product may be returned to 36°F to 46°F (2°C to 8°C) up to the expiration date. Dispose of syringes if stored at room temperature for more than 5 days.
Avoid shaking. The solution should be visually inspected prior to use. Only clear solutions without particles should be used. Exposure to 23°F to 30°F (-1°C to -5°C) for up to 72 hours and temperatures as low as 5°F to -13°F (-15°C to -25°C) for up to 24 hours do not adversely affect the stability of GRANIX.
Single-dose syringe and single-dose vial – discard unused portion. Any unused product or waste material should be disposed of in accordance with local requirements.
If GRANIX gets on the skin, wash the area with soap and water. If GRANIX gets in the eyes, thoroughly flush the exposed eye/eyes with water.
-
17 PATIENT COUNSELING INFORMATION
Availability of Patient Information and Instructions for Use
Advise all patients and/or caregivers to read the FDA-approved Patient Information. For patients that are candidates for self-administration, assist patients and caregivers in understanding the contents of the Patient Information as well as the GRANIX Instructions for Use that are included with the product, and give them the opportunity to ask questions prior to initiating therapy.
Patient Training
Once it is determined that a patient is an appropriate candidate for self-administration or administration by a caregiver, instruct the patient or caregivers on the proper storage, preparation, and administration technique for GRANIX. Patients should be advised not to skip or change their dose or stop taking GRANIX without talking to their healthcare provider first. Advise the patients to read the FDA-approved Patient Information and Instructions for Use for further information.
Bone Pain
Bone pain is common. Analgesics such as acetaminophen or NSAIDS may be necessary [see Adverse Reactions (6.1)].
Rupture or Enlargement of Spleen
Rupture or enlargement of the spleen may occur, which may be signaled by abdominal pain, left upper quadrant pain, or left shoulder pain. Advise patients to report onset of pain in these areas to their doctor immediately [see Warnings and Precautions (5.1)].
Dyspnea
Dyspnea with or without fever, progressing to Acute Respiratory Distress Syndrome, may occur. Advise patients to report dyspnea immediately to their doctor [see Warnings and Precautions (5.2)].
Allergic Reactions
Serious allergic reactions, including anaphylaxis, rash, and urticaria: Patients should report such reactions immediately to their doctor [see Warnings and Precautions (5.3)].
Sickle Cell Disorders
In patients with sickle cell disorders, sickle cell crisis and death has occurred. Discuss the potential risks and benefits for patients with sickle cell disorders prior to the administration of GRANIX [see Warnings and Precautions (5.4)].
Glomerulonephritis
Symptoms may include swelling of the face or ankles, dark colored urine or blood in the urine, or a decrease in urine production. Advise patients to report signs or symptoms of glomerulonephritis to their physician immediately [see Warnings and Precautions (5.5)].
Myelodysplastic Syndrome and Acute Myeloid Leukemia
There may be an increased risk of Myelodysplastic Syndrome and/or Acute Myeloid Leukemia in patients with congenital neutropenia who receive GRANIX and in patients with breast and lung cancer who receive GRANIX in conjunction with chemotherapy and/or radiation therapy. Symptoms of MDS and AML may include tiredness, fever, and easy bruising or bleeding. Advise patients to report to their physician signs and symptoms of MDS/AML [see Warnings and Precautions (5.8)].
Pregnancy
Inform patients not to become pregnant while receiving GRANIX. If pregnancy occurs, advise patients of the possibility of fetal harm [see Use in Specific Populations (8.1)].
Lactation
Advise women to avoid breastfeeding during treatment with GRANIX and for 2 weeks after the final dose. [see Use in Specific Populations (8.2)].
See FDA-approved Patient Labeling (Patient Information) and Instructions for Use.
TBO-010

©2023 Cephalon, Inc., a wholly owned subsidiary of Teva Pharmaceutical Industries Ltd. All rights reserved.
Manufactured by:
UAB Teva Baltics
Vilnius, LithuaniaU.S. License No. 1803
Distributed by:
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454Product of Israel
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
GRANIX (GRAN-icks)
(tbo-filgrastim)
injection, for subcutaneous useWhat is GRANIX? GRANIX is a prescription medicine:
- used in adults and children 1 month of age and older with certain types of cancer (non-myeloid malignancies), who are receiving chemotherapy that affects the bone marrow
- given to help decrease the length of time that the number of certain white blood cells (neutrophils) are very low (severe neutropenia). Neutrophils are white blood cells that are important in fighting bacterial infections.
Do not take GRANIX if you have had a serious allergic reaction to filgrastim products or pegfilgrastim products. Before you receive GRANIX, tell your healthcare provider about all of your medical conditions, including if you: - have a sickle cell disorder
- have kidney problems
- plan to have bone scans or tests
- are pregnant or plan to become pregnant. It is not known if GRANIX will harm your unborn baby. You should not become pregnant during treatment with GRANIX.
- are breastfeeding or plan to breastfeed. It is not known if GRANIX passes into your breast milk. Do not breastfeed during treatment with GRANIX and for 2 weeks after the final dose.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How will I receive GRANIX? - GRANIX is given by an injection under your skin (subcutaneous) by a healthcare provider. Your healthcare provider may decide injections can be given at home by you or your caregiver. If GRANIX is given at home, see the detailed “Instructions for Use” that comes with your GRANIX for information on how to prepare and inject a dose of GRANIX.
- Your healthcare provider will show you and your caregiver how to prepare and inject GRANIX before you use it.
- Your healthcare provider will tell you how much GRANIX to inject and when to inject it. Do not stop using GRANIX or change your dose unless your healthcare provider tells you to.
- GRANIX injections are usually given 1 time each day until your white blood cell count returns to normal.
- Your first dose of GRANIX is given at least 24 hours after you receive your chemotherapy.
- Do not inject GRANIX within 24 hours before your next dose of chemotherapy.
- Your healthcare provider will test your blood before your chemotherapy and during treatment with GRANIX.
- If GRANIX gets on your skin or your caregiver’s skin, wash the area with soap and water.
- If GRANIX gets in your eyes or your caregiver’s eyes, flush the eyes well with water.
What are the possible side effects of GRANIX? GRANIX can cause serious side effects, including:
- Spleen rupture. Your spleen may become enlarged and can rupture. A ruptured spleen can cause death. Call your healthcare provider right away if you have pain in your left upper stomach (abdomen)-area or your left shoulder during treatment with GRANIX.
- A serious lung problem called acute respiratory distress syndrome (ARDS). Call your healthcare provider or get emergency medical help right away if you have shortness of breath with or without fever, trouble breathing, or a fast rate of breathing.
- Serious allergic reactions. GRANIX can cause serious allergic reactions. These reactions can cause a rash over your whole body, shortness of breath, wheezing, dizziness, swelling around your mouth or eyes, fast heart rate, and sweating. If you have any of these symptoms, stop using GRANIX and call your healthcare provider or get emergency help right away.
- Sickle cell crisis. You may have a serious sickle cell crisis, which could lead to death, if you have a sickle cell disorder and use GRANIX. Call your healthcare provider right away if you have symptoms of sickle cell crisis such as pain or difficulty breathing.
-
Kidney injury (glomerulonephritis). GRANIX can cause kidney injury. Call your healthcare provider right away if you develop any of the following symptoms:
- swelling of your face or ankles
- blood in your urine or dark colored urine
- you urinate less than usual
- Increased white blood cell count (leukocytosis). Your healthcare provider will check your blood during treatment with GRANIX.
-
Capillary Leak Syndrome. GRANIX can cause fluid to leak from blood vessels into your body’s tissues. This condition is called “Capillary Leak Syndrome” (CLS). CLS can quickly cause you to have symptoms that may become life-threatening. Get emergency medical help right away if you develop any of the following symptoms:
- swelling or puffiness and are urinating less than usual
- trouble breathing
- swelling of your stomach-area (abdomen) and feeling of fullness
- dizziness or feeling faint
- a general feeling of tiredness
-
Myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML).
- GRANIX may increase the risk of developing a precancerous condition called MDS or a type of blood cancer called AML in people who were born with low white blood cell counts (congenital neutropenia).
- If you have breast cancer or lung cancer, when GRANIX is used with chemotherapy and radiation therapy, or with radiation therapy only, you may have an increased risk of developing MDS or AML.
- Symptoms of MDS and AML may include tiredness, fever, and easy bruising or bleeding.
- Call your healthcare provider if you develop any of these symptoms during treatment with GRANIX.
- Inflammation of the aorta (aortitis). Inflammation of the aorta (the large blood vessel which transports blood from the heart to the body) has been reported in patients who received another filgrastim product. Symptoms may include fever, abdominal pain, feeling tired, and back pain. Call your healthcare provider if you experience these symptoms.
Your healthcare provider may decrease your dose, temporarily stop, or permanently stop treatment with GRANIX if you have certain side effects.
The most common side effect of GRANIX is bone pain.
The most common side effects of GRANIX in children include:
- decreased platelet count
- fever
- pain in the arms and legs
- headache
- diarrhea
These are not all of the possible side effects of GRANIX.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store GRANIX? - Store GRANIX in the refrigerator between 36˚F to 46˚F (2˚C to 8˚C).
- Keep GRANIX away from light to protect it. If your GRANIX prefilled syringe or vial comes in a carton, keep it in the carton until you are ready to use it, to protect from light.
- Do not shake.
- Take GRANIX out of the refrigerator 30 minutes before use and allow it to reach room temperature before preparing an injection.
- GRANIX can be left at room temperature for up to 5 days. If not used within 5 days, return GRANIX back to the refrigerator. Throw away (dispose of) GRANIX that has been left at room temperature for more than 5 days.
- After you inject your dose, throw away (dispose of) any unused GRANIX left in the syringe or vial. Do not save unused GRANIX in the syringe or the vial for later use.
Keep GRANIX and all medicines out of the reach of children.
General information about the safe and effective use of GRANIX Medicines are sometimes prescribed for purposes other than those listed in Patient Information leaflets. Do not use GRANIX for a condition for which it was not prescribed. Do not give GRANIX to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about GRANIX that is written for health professionals.
What are the ingredients in GRANIX? Active ingredient: tbo-filgrastim
Inactive ingredients: glacial acetic acid, sorbitol, polysorbate 80, sodium hydroxide, and Water for Injection
Manufactured by:
UAB Teva Baltics
Vilnius, LithuaniaU.S. License No. 1803
Distributed by:
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454Product of Israel

TBOPL-008
©2023 Cephalon, Inc., a wholly owned subsidiary of Teva Pharmaceutical Industries Ltd. All rights reserved.
For more information, call 1-800-896-5855.
This Patient Information has been approved by the U.S. Food and Drug Administration Revised: 11/2023
-
INSTRUCTIONS FOR USE - Prefilled Syringe
GRANIX (GRAN-icks)
(tbo-filgrastim)
for subcutaneous injection
Single-Dose Prefilled SyringeImportant:
Read the Prescribing information and Patient Package insert for important information about GRANIX.
About the GRANIX syringe
Depending on the prescription that your healthcare provider gave you, you will receive a syringe that provides a dose of either 0.1 mL to 0.5 mL or 0.1 mL to 0.8 mL. If you are prescribed a dose over 0.8 mL, two syringes will be required to reach your prescribed dose. Your healthcare provider will determine how many syringes and the correct dose in milliliters (mL) you will need to give based on your body weight. You should continue to give GRANIX daily until your healthcare provider informs you that your white blood cell count has returned to normal.
Make sure you understand the following:
- How to store your syringes.
- How to read the syringe markings.
- How to adjust the amount of GRANIX in the syringe for your prescribed dose.
- How to prepare and give the injection.
Do not shake syringes.
Do not remove the needle cap until you are ready to inject.
Do not re-use a syringe. The syringe is for single-use only.
Your first dose of GRANIX is given at least 24 hours after you receive your chemotherapy.
Do not inject GRANIX within 24 hours before your next dose of chemotherapy.
Dosing schedule
Inject your total daily dose 1 time each day as prescribed by your healthcare provider, starting at least 24 hours (1 day) after the end of your chemotherapy cycle.
You should continue to give GRANIX daily until your white blood cell count returns to normal.
How to store your GRANIX syringes
- Store GRANIX in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Store GRANIX in the original carton to protect it from light.
- Do not shake.
- Take GRANIX out of the refrigerator 30 minutes before use and allow it to reach room temperature before preparing an injection.
- GRANIX syringes can be left at room temperature for a single period of up to 5 days, and if not used can be returned to the refrigerator. Throw away (dispose of) any GRANIX syringes that have been left at room temperature for more than 5 days.
- After you inject your dose, throw away (dispose of) any unused GRANIX left in the syringe. Do not save unused GRANIX in the syringe for later use.
Keep GRANIX and all medicines out of the reach of children.
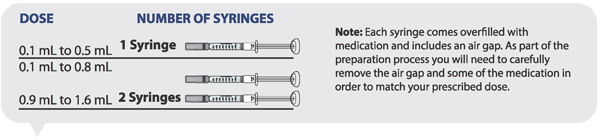
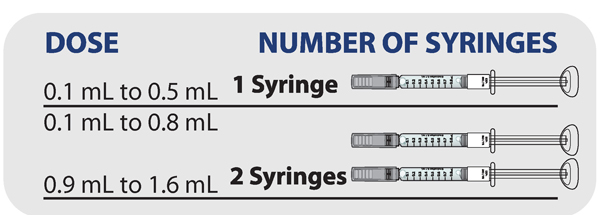
Determining how many syringes you need for your daily dose
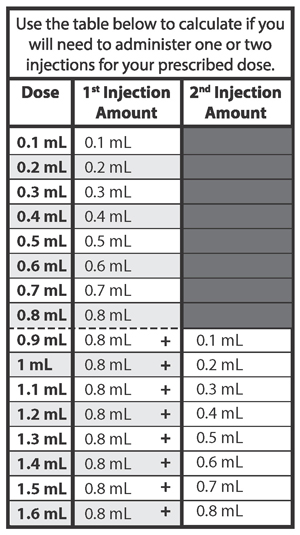
- If your prescribed daily dose is 0.5 mL or less, use 1 syringe.
- If your prescribed daily dose is 0.8 mL or less, use 1 syringe.
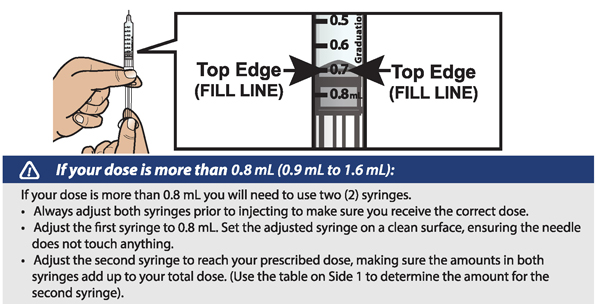
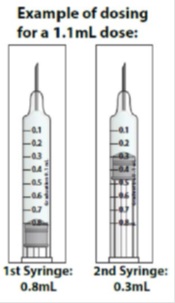
- If your prescribed daily dose is more than 0.8 mL you will need to prepare 2 syringes in order to match your prescribed dose:
- Adjust your first syringe to 0.8 mL.
- Adjust your second syringe to the additional amount required to make up your total prescribed dose.
- Make sure the amounts in both syringes add up to your prescribed dose (See the table to the right to determine how much medicine should be in each syringe).
For example: If your prescribed dose is 1 mL you would prepare 1 syringe with 0.8 mL and a second syringe with 0.2 mL.
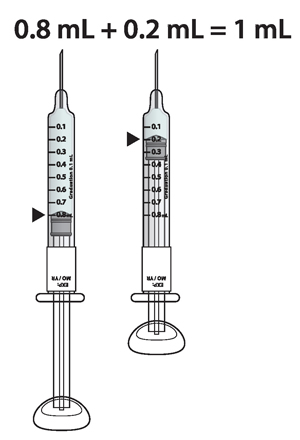
Important: When using 2 syringes always adjust the first syringe to 0.8 mL.
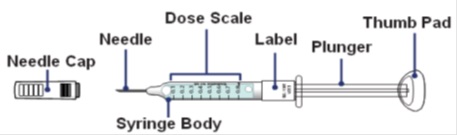
How to read the syringe markings
What the markings on the syringe mean:
The syringe is labeled in 0.1 mL unit increments from 0.1 mL to 0.8 mL. There is a line next to each 0.1 mL unit increment.
To read the dose scale always hold the syringe with the needle-end facing up so that 0.1 mL is at the top and 0.8 mL is at the bottom.
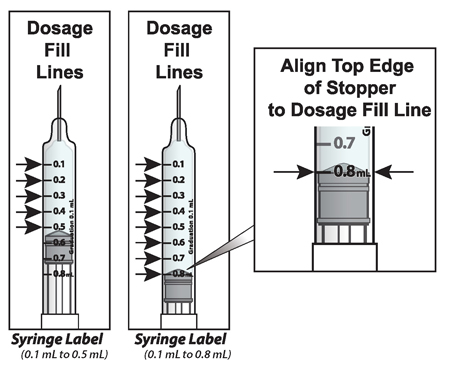
How to adjust the medicine level for your prescribed dose
- When setting your dose, (See 2C) you will line up the top edge of the grey rubber stopper with the line on the syringe scale that matches your prescribed dose.
- Note: The top edge of the grey rubber stopper is the edge directly below the dome at the top of the stopper.
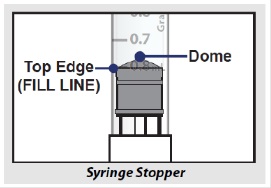
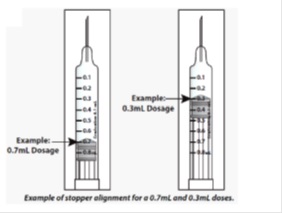
Do not use the top of the cone or the middle or lower edges of the grey stopper to measure your dose.
Injection steps (follow the steps below for each day of dosing)
1. Prepare for injection
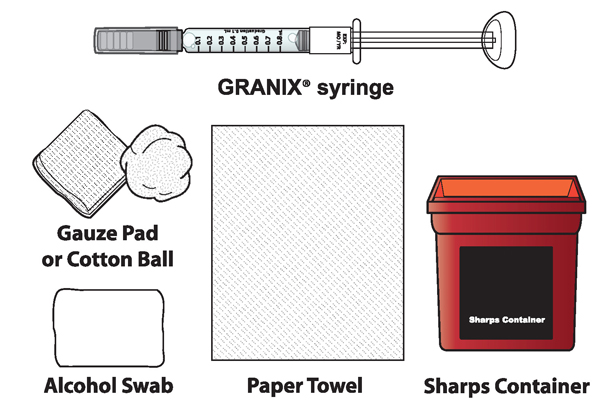
1A Each time you inject a dose gather the following supplies:
- GRANIX syringe(s)
- Alcohol swabs
- Paper towel
- Cotton ball or gauze pad
- Adhesive Bandage, if needed
- Sharps disposal container (hard-walled container for discarding syringes)
Note: GRANIX and all of the parts of the prefilled syringe do not contain natural rubber latex.
1B Take the carton with the syringe(s) out of the refrigerator
1C Check the label and the expiration date on the side of the carton

Important: Do not inject if:
- “GRANIX® (tbo-filgrastim)” is not listed on the carton.
- The expiration date on the syringe label has passed.
1D Remove the syringe(s) from the carton
Open the carton by breaking the tamper proof seal and lifting the lid. Remove the number of syringes required for your daily dose by grasping each at the middle of the syringe body.
After removing your required number of syringes, place the carton back in the refrigerator.
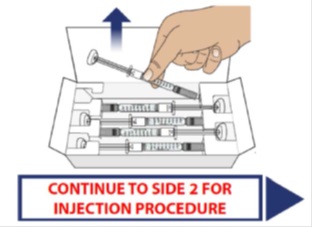
1E Look carefully at the syringe(s) and the medicine
Hold the syringe body and check to make sure it is not damaged.
Inspect the medicine in the syringe. GRANIX should be a clear liquid.
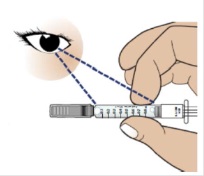
Important: Do not inject if:
- GRANIX® (tbo-filgrastim) is not listed on the syringe label.
- The medicine is cloudy, discolored, or foamy.
- The medicine contains lumps, flakes, or particles.
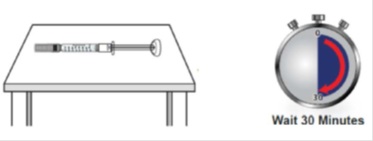
1F Wait 30 minutes for the syringe(s) to warm to room temperature
Wait 30 minutes for GRANIX to naturally warm to room temperature. This will provide a more comfortable injection.
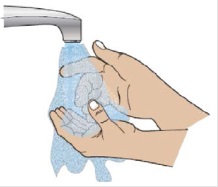
1G Wash your hands
When ready to inject, wash your hands with soap and warm water and dry thoroughly with a clean towel.
1H Choose an injection site
The recommended injection sites are:
If you are self-injecting:
Stomach-area (abdomen): Except for a 2-inch area around the navel (belly button).
Thighs: Top or middle area of thighs.
If a caregiver is injecting GRANIX for you:
Arms: Fleshy areas on upper, back part of the arm.
Upper hip or buttock: Fleshy areas around the back of the upper hips and upper sides of the buttocks.
If 2 injections will be performed, then the second injection should be at least 1 inch away from the first injection.
Choose Injection Site
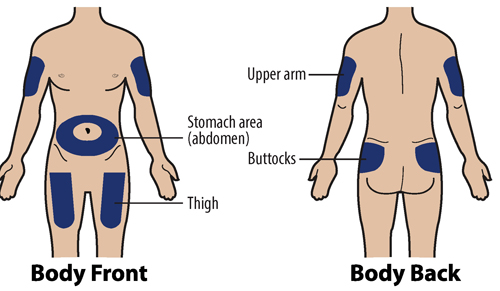
Do not inject into areas that are tender, red, bruised, hard, or have scars or stretch marks.
Important:
- You should select a different injection site each time you give yourself an injection.
- If you want to use the same injection site for a dose requiring 2 injections, make sure the second injection site is at least 1 inch away from the first injection site.
1I Clean the injection site using an alcohol swab
Allow site to dry for 5-10 seconds to avoid stinging.
If giving 2 injections, then the distance between the 2 injection sites should be at least 1 inch apart.
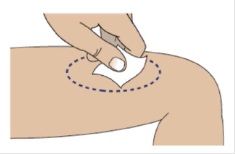
Do not touch or blow on site after cleaning.
2. Adjust medicine level for your prescribed dose
2A Remove the needle cap from the syringe
Place a paper towel on the table.
To remove the needle cap, hold the body of the syringe firmly with 1 hand (with the needle facing away from you).
Pull the needle cap straight off, extending your hand away from the needle.
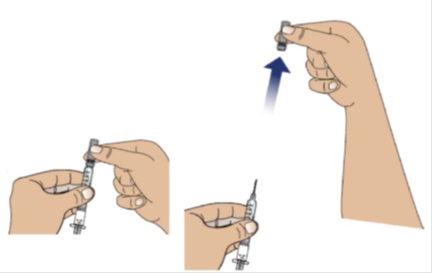
Note: Throw away the needle cap in a sharps disposal container.
Do not recap the needle now or after the injection.
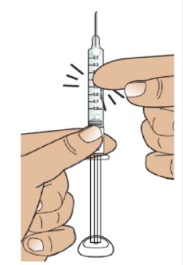
2B Hold the syringe upright and tap
Hold the syringe upright (needle pointing up), as shown.
Gently tap the barrel with your fingers to make sure any air bubbles rise to the top.
2C Slowly and carefully adjust the medicine level
Hold the syringe with the needle pointing up and slightly away from you, as shown. Make sure you can easily see the syringe markings and numbers.
Holding the plunger as shown, very slowly and carefully push the plunger up until the top edge of the grey rubber stopper is even with the line that corresponds to your prescribed dose.
Note:
- If GRANIX gets on your skin, wash your skin with soap and water.
- If GRANIX get in your eyes, flush well with water.
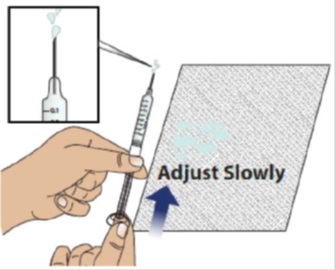
Note: If you accidentally removed too much GRANIX, contact your healthcare provider before giving your injection.
3. INJECT MEDICATION
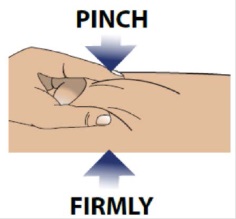
3A Pinch skin
The illustration below is an example only
Use your free hand to firmly pinch the skin you previously cleaned.
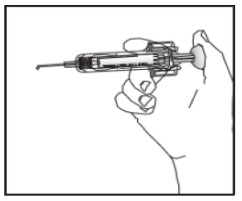
3B Insert the needle at a 45 to 90 degree angle
Hold the body of the syringe between your thumb and index finger.
Use a quick motion to fully insert the needle straight into the pinched skin at a 45 to 90 degree angle.
When the needle is inserted, you can release the pinched skin.
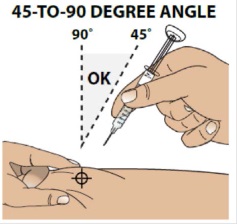
Do not hold or push on the plunger while inserting the needle into the skin.
3C Push the plunger down to inject all of the GRANIX
Use your finger to gently push down on the plunger.
When the plunger head is as far down as it will go, all of the GRANIX has been injected. When done, gently remove the needle from the skin.
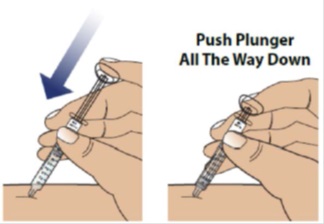
3D Throw away (dispose of) used syringe
Put your used needles and syringes in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash.
If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
3E Treat the injection site if needed and wash your hands
If you see drops of blood at the injection site, you can press a cotton ball or gauze over the injection site for several seconds to stop the bleeding.
Apply bandage, if needed.
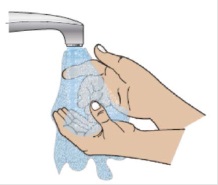
When you are finished, wash your hands with soap and warm water and dry thoroughly with a clean towel.
4. Repeat the injection steps with the second syringe (If dose is more than 0.8 mL)
If your dose is more than 0.8 mL:
- Follow instructions 3A through 3E for injecting.
- Choose a different site for your second injection. If you want to use the same part of your body, make sure the second injection site is at least 1 inch away from the first injection site.
If you have any questions or concerns about your dose of GRANIX or how to prepare and give your injections, call your healthcare provider.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
UAB Teva Baltics
Vilnius, LithuaniaU.S. License No. 1803
Distributed by:
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454Product of Israel
Revised: 11/2023
TBOIFU-004

©2023 Cephalon, Inc., a wholly owned subsidiary of Teva Pharmaceutical Industries Ltd. All rights reserved.
GRANIX is a trademark of Teva Pharmaceutical Industries Ltd.
-
INSTRUCTIONS FOR USE - VIAL
INSTRUCTIONS FOR USE
GRANIX (GRAN-icks)
(tbo-filgrastim)
for subcutaneous injection
Single-Dose VialImportant:
Read the Prescribing information and Patient Package insert for important information about GRANIX.
Before you use a GRANIX vial, read this important information:
- GRANIX vial is supplied as either 300 mcg in 1 mL solution, or 480 mcg of tbo-filgrastim in 1.6 mL solution. Your healthcare provider will determine which strength of GRANIX to prescribe for you. Your healthcare provider will prescribe the correct number of vials, and the dose in milliliters (mL) that you will need to inject based on your body weight.
- When you receive your vials of GRANIX at the pharmacy, check the label to be sure that the dose strength on the vial matches the dose strength that your healthcare provider prescribed for you. If you are not sure, ask your pharmacist.
- If you are told that more than 1 injection is needed for each dose of GRANIX, the total dose should be divided into two equal parts. Each of the two parts of your dose should be drawn from a separate vial.
- Your healthcare provider will show you how to measure the correct dose of GRANIX before you try to inject it for the first time. This dose will be measured in milliliters (mL).
How to store your GRANIX vial
- Store GRANIX in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Keep GRANIX vials away from light to protect it. If your GRANIX vial comes in a carton, keep it in the carton until you are ready to use it to protect from light.
- Do not freeze.
- Take GRANIX out of the refrigerator 30 minutes before use and allow it to reach room temperature before preparing an injection.
- GRANIX vials can be left at room temperature for a single period of up to 5 days, and if not used can be returned to the refrigerator to use later. Throw away (dispose of) any GRANIX vials that have been left at room temperature for more than 5 days.
- After you inject your dose, properly dispose of any unused GRANIX left in the vial. Do not save unused GRANIX for later use.
Keep GRANIX and all medicines out of the reach of children.
Using your vial
- It is important that you do not try to give the injection unless you or your caregiver has received training from your healthcare provider.
- Make sure that the name GRANIX appears on the carton and vial label.
- Check the label and the expiration date on the side of the carton. Do not use a vial after the expiration date on the label.
- Do not shake the vial.
- Do not use the vial if the medicine is cloudy or discolored, or contains flakes or particles.
Do not inject your first dose of GRANIX until at least 24 hours after you receive your chemotherapy. You should continue to receive GRANIX daily until your healthcare provider tells you that your white blood cell count has returned to normal. Do not inject GRANIX less than 24 hours before your next dose of chemotherapy.
Call your healthcare provider if you have any questions.
Follow the steps below for each day of dosing
STEP 1: Prepare
Step 1A: Remove GRANIX from the refrigerator
Take the GRANIX® (tbo-filgrastim) carton out of the refrigerator. Open the carton by lifting the lid and breaking the seal.
Step 1B: Check the label and the expiration date on the carton
- Check to make sure "GRANIX® (tbo-filgrastim)” is listed on the carton
- Do not use if the expiration date on the carton has passed
Remove the number of vials needed for the daily dose.
Return the carton containing any unused vials to the refrigerator.
Step 1C: Wait 30 minutes for the vials to reach room temperature.
Place the vials of GRANIX on a clean, well-lit flat work surface for about 30 minutes to warm to room temperature. This will help to provide a more comfortable injection.
- Do not try to warm the vial by using a heat source such as hot water or microwave
- Protect the vial from light
- Do not shake the vial
- Use a vial only 1 time.
Step 1D: Inspect the vial
Hold each vial and check to make sure it is not damaged.
Inspect the medicine in the vial. Make sure the medicine in the vial is clear and colorless.
- Check to make sure GRANIX is listed on the vial label.
- Do not use the vial if:
- The medicine is cloudy or discolored, or contains flakes or particles.
- The expiration date on the vial label has passed
- In these cases, use a new vial and call your healthcare provider.
Step 1E: Gather the following supplies needed for each injection and place them on your clean work surface (Figure A):
- 1 GRANIX vial
- 1 disposable syringe and needles
- 2 alcohol swabs
- 1 cotton balls or gauze pad(s)
- 1 adhesive bandage(s), if needed
- 1 sharps disposal container
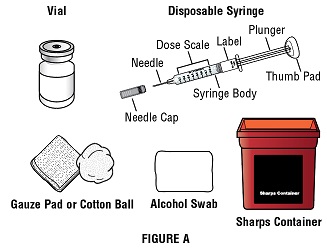
- Only use disposable syringes and needles that your healthcare provider prescribes.
- Only use the syringes and needles 1 time. Throw away (dispose of) any used syringes and needles in a sharps disposal container.
- You should only use syringes that are marked in tenths of milliliters (mL).
- Your healthcare provider will show you how to measure the correct dose of GRANIX. This dose will be measured in milliliters (mL).
Step 1F: Wash your hands.
When ready to inject, wash your hands well with soap and water, and dry with a clean towel.
Step 2: Get ready
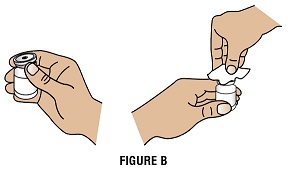
Step 2A: Take the cap off the vial (Figure B). Clean the rubber stopper with 1 alcohol swab.
Step 2B: Check the packaging for the syringe and needle. If the packaging has been open or damaged, do not use that syringe and needle. Throw away (dispose of) that syringe and needle in your sharps disposal container.
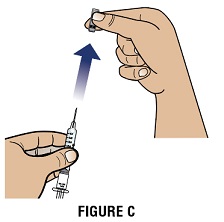
Step 2C: Hold the syringe by the barrel with the needle cap pointing up. Carefully pull the needle cap straight off and away from your body (Figure C).
Important: Throw away (Dispose of) the needle cap.
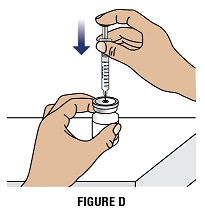
Step 2D: Keep the vial on the flat work surface and insert the needle straight down through the rubber stopper on top of the vial. Do not insert the needle through the rubber stopper more than 1 time (Figure D).
Step 2E: Push the plunger down and inject all the air from the syringe into the vial of GRANIX (Figure D)
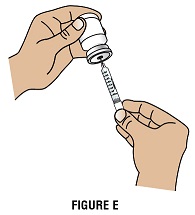
Step 2F: Keep the needle in the vial and turn the vial upside down. Make sure that the GRANIX liquid is covering the tip of the needle (Figure E).
Step 2G: Keep the vial upside down and slowly pull back on the plunger to fill the syringe barrel with GRANIX to the correct marking amount (mL) of medicine that matches the dose your healthcare provider prescribed.
Step 2H: Keep the needle in the vial and check for air bubbles in the syringe. If there are air bubbles, gently tap the syringe barrel with your finger until the air bubbles rise to the top. Slowly push the plunger up to push the air bubbles out of the syringe (Figure F).
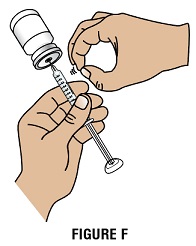
Step 2I: Keep the tip of the needle in the liquid and pull the plunger back to the number on the syringe barrel that matches your dose. Check again for air bubbles. The air in the syringe will not hurt you, but too large an air bubble can reduce your dose of GRANIX. If there are still air bubbles, repeat the steps above to remove them.
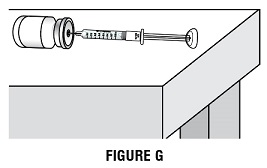
Step 2J: Check again to make sure that you have the correct dose in the syringe. It is important that you use the exact dose prescribed by your healthcare provider. Do not remove the needle from the vial. Lay the vial down on its side with the needle still in the vial while you prepare the injection site (Figure G).
Step 3 – Select and prepare the injection site
Step 3A: Choose an injection site (Figure H)
You can use:
- Stomach-area (abdomen): Except for a 2-inch area around the navel (belly button)
-
Thighs: Top or middle area of thighs
-
Arms: Fleshy areas on upper, back part of the arm (only if someone else is giving you the injection)
-
Upper outer area of your buttocks: Fleshy areas around the back of the upper hips and upper sides of the buttocks (only if someone else if giving you the injection).
- If 2 injections will be performed, then the second injection should be at least 1 inch away from the first injection.
Choose Injection Site
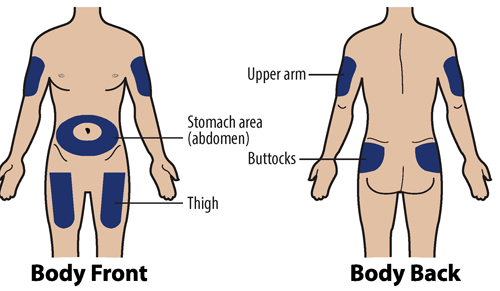
Figure H
Do not inject into areas that are tender, red, bruised, hard, or have scars or stretch marks.
Important:
You should select a different injection site each time you give yourself an injection.
If you want to use the same injection site for a dose requiring 2 injections, make sure the second injection site is at least 1 inch away from the first injection site.
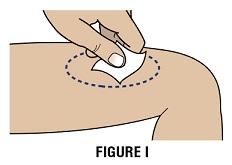
Step 3B: Clean the injection site using a new alcohol swab (Figure I).
Allow site to dry for 5-10 seconds to avoid stinging.
If giving 2 injections, then the distance between the 2 injection sites should be at least 1 inch apart.
-
Do not touch this area again before injecting.
Step 4 –Inject medication
Step 4A: Remove the prepared syringe and needle from the vial.
Step 4B: With your other hand, pinch the skin around the injection site to create a firm surface (Figure J).
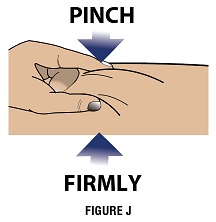
Important: Keep skin pinched while inserting the needle.
Step 4C: Insert the needle at a 45 to 90 degree angle (Figure K)
-
Hold the body of the syringe between your thumb and index finger.
-
Use a quick motion to fully insert the needle straight into the pinched skin at a 45 to 90 degree angle.
- When the needle is inserted, you can release the pinched skin.
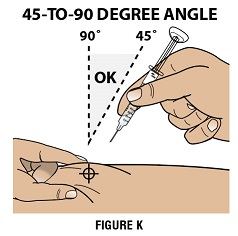
- Do not hold or push on the plunger while inserting the needle into the skin.
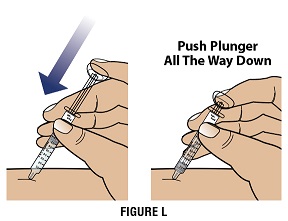
Step 4D Push the plunger down to inject all of the GRANIX (Figure L)
-
Use your finger to gently push down on the plunger.
-
When the plunger head is as far down as it will go, all of the GRANIX has been injected. When done, gently remove the needle from the skin.
Step 4E: Throw away (dispose of) used needle and syringe
- Put your used needles and syringes in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Step 4F: Treat the injection site if needed and wash your hands.
If you see drops of blood at the injection site, you can press a cotton ball or gauze over the injection site for several seconds to stop the bleeding.
Apply bandage, if needed.
When you are finished, wash your hands with soap and water (Figure M).
Step 4G: Repeat steps 1E through 4F with a new vial of GRANIX if your healthcare provider instructs you that your dose is more than 1 vial.
If you have any questions or concerns about your dose of GRANIX or how to prepare and give your injections, call your healthcare provider.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
UAB Teva Baltics
Vilnius, LithuaniaU.S. License No. 1803
Distributed by:
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454Product of Israel
Revised: 11/2023
TBOIFUV-003

©2023 Cephalon, Inc., a wholly owned subsidiary of Teva Pharmaceutical Industries Ltd. All rights reserved.
GRANIX is a trademark of Teva Pharmaceutical Industries Ltd.
-
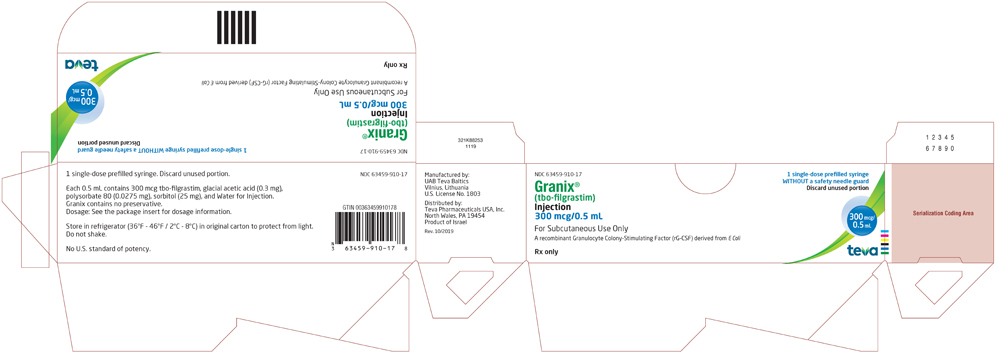
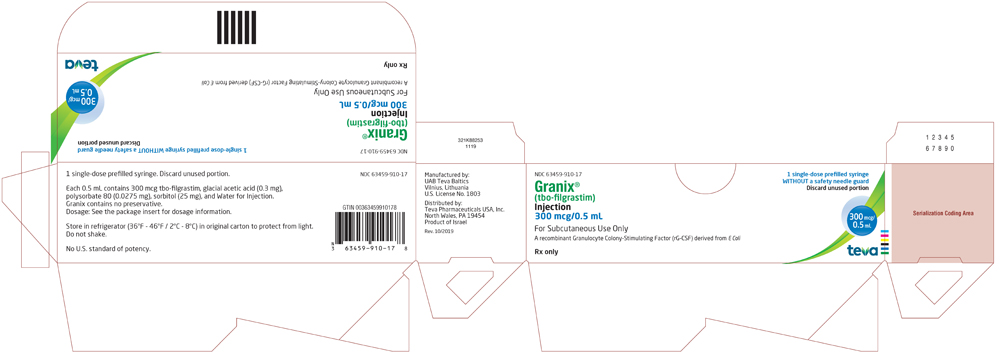
Package/Label Display Panel 63459-910-11
NDC 63459-910-11
Rx only
Granix® (tbo-filgrastim) Injection
300 mcg/0.5 mL
For Subcutaneous Use Only
A recombinant Granulocyte Colony-Stimulating Factor (rG-CSF) derived from E Coli
300 mcg/0.5 mL
For Healthcare Professional Use Only
1 Single-dose prefilled syringe with a safety needle guard
Discard unused portion

- Package/Label Display Panel, 63459-910-17
-
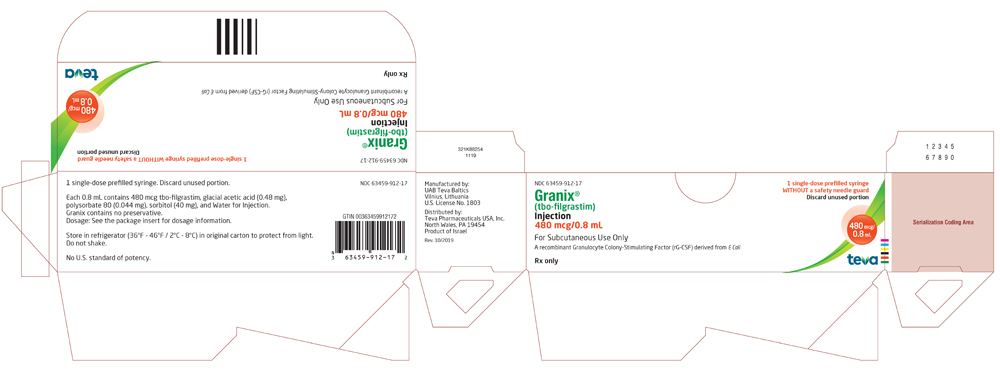
Package/Label Display Panel 63459-912-11
NDC 63459-912-11
Rx only
Granix® (tbo-filgrastim) Injection
480 mcg/0.8 mL
For Subcutaneous Use Only
A recombinant Granulocyte Colony-Stimulating Factor (rG-CSF) derived from E Coli
480 mcg/0.8 mL
For Healthcare Professional Use Only
1 Single-dose prefilled syringe with a safety needle guard
Discard unused portion

-
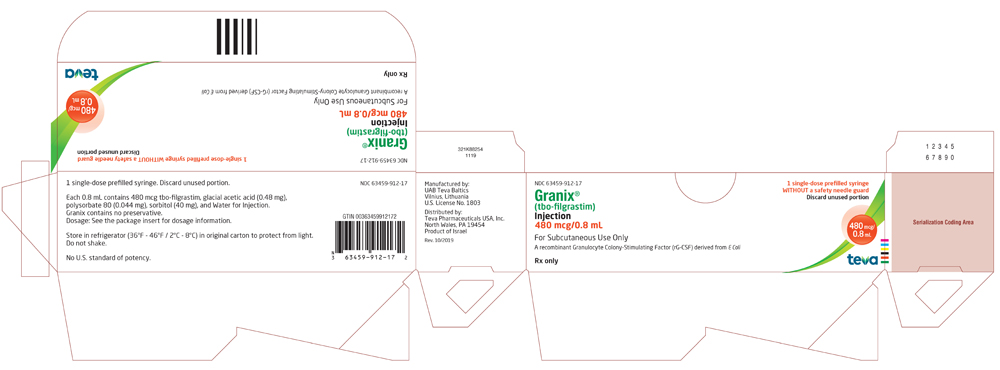
Package/Label Display Panel, 63459-912-17
NDC 63459-912-17
Rx only
Granix® (tbo-filgrastim) Injection
480 mcg/0.8 mL
For Subcutaneous Use Only
A recombinant Granulocyte Colony-Stimulating Factor (rG-CSF) derived from E Coli
480 mcg/0.8 mL
For Healthcare Professional Use Only
1 Single-dose prefilled syringe WITHOUT a safety needle guard
Discard unused portion
- Package/Label Display Panel, 63459-918-59
- Package/Label Display Panel, 63459-920-59
-
INGREDIENTS AND APPEARANCE
GRANIX
tbo-filgrastim injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63459-910 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FILGRASTIM (UNII: PVI5M0M1GW) (FILGRASTIM - UNII:PVI5M0M1GW) FILGRASTIM 300 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength ACETIC ACID (UNII: Q40Q9N063P) SORBITOL (UNII: 506T60A25R) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63459-910-11 1 in 1 CARTON 11/11/2013 1 NDC:63459-910-12 1 in 1 BLISTER PACK 1 NDC:63459-910-01 0.5 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC:63459-910-15 10 in 1 CARTON 11/11/2013 2 NDC:63459-910-12 1 in 1 BLISTER PACK 2 NDC:63459-910-01 0.5 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 3 NDC:63459-910-17 1 in 1 CARTON 03/02/2015 3 NDC:63459-910-18 0.5 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 4 NDC:63459-910-36 5 in 1 CARTON 03/02/2015 4 NDC:63459-910-18 0.5 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125294 11/11/2013 GRANIX
tbo-filgrastim injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63459-912 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FILGRASTIM (UNII: PVI5M0M1GW) (FILGRASTIM - UNII:PVI5M0M1GW) FILGRASTIM 480 ug in 0.8 mL Inactive Ingredients Ingredient Name Strength ACETIC ACID (UNII: Q40Q9N063P) SORBITOL (UNII: 506T60A25R) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63459-912-11 1 in 1 CARTON 11/11/2013 1 NDC:63459-912-12 1 in 1 BLISTER PACK 1 NDC:63459-912-01 0.8 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC:63459-912-15 10 in 1 CARTON 11/11/2013 2 NDC:63459-912-12 1 in 1 BLISTER PACK 2 NDC:63459-912-01 0.8 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 3 NDC:63459-912-17 1 in 1 CARTON 03/02/2015 3 NDC:63459-912-18 0.8 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 4 NDC:63459-912-36 5 in 1 CARTON 03/02/2015 4 NDC:63459-912-18 0.8 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125294 11/11/2013 GRANIX
tbo-filgrastim injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63459-918 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FILGRASTIM (UNII: PVI5M0M1GW) (FILGRASTIM - UNII:PVI5M0M1GW) FILGRASTIM 300 ug in 1 mL Inactive Ingredients Ingredient Name Strength ACETIC ACID (UNII: Q40Q9N063P) SORBITOL (UNII: 506T60A25R) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63459-918-59 10 in 1 CARTON 11/07/2018 1 NDC:63459-918-53 1 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125294 11/07/2018 GRANIX
tbo-filgrastim injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63459-920 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FILGRASTIM (UNII: PVI5M0M1GW) (FILGRASTIM - UNII:PVI5M0M1GW) FILGRASTIM 480 ug in 1.6 mL Inactive Ingredients Ingredient Name Strength ACETIC ACID (UNII: Q40Q9N063P) SORBITOL (UNII: 506T60A25R) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63459-920-59 10 in 1 CARTON 11/07/2018 09/30/2026 1 NDC:63459-920-53 1.6 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125294 11/07/2018 Labeler - Cephalon, LLC (183236314)