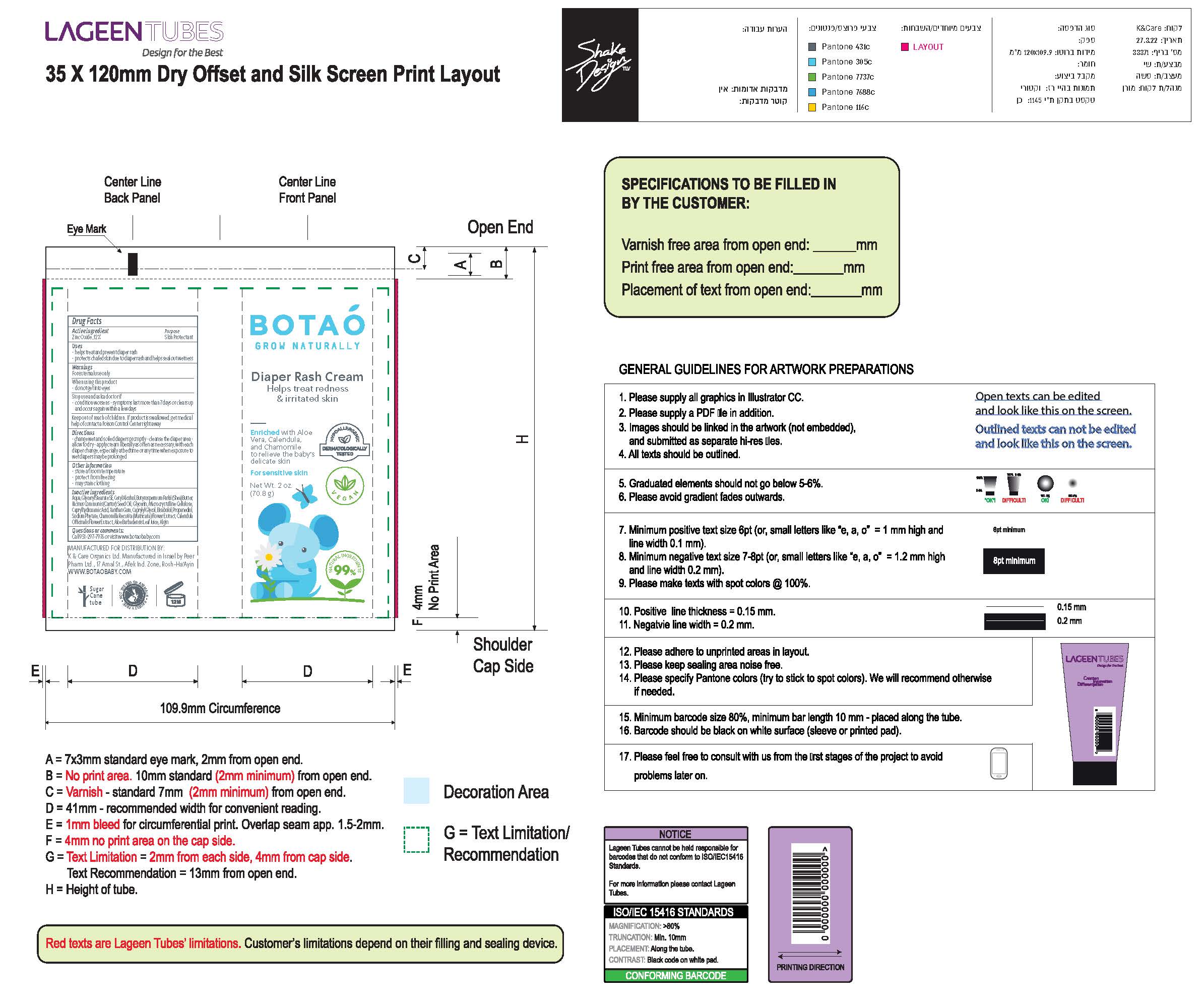
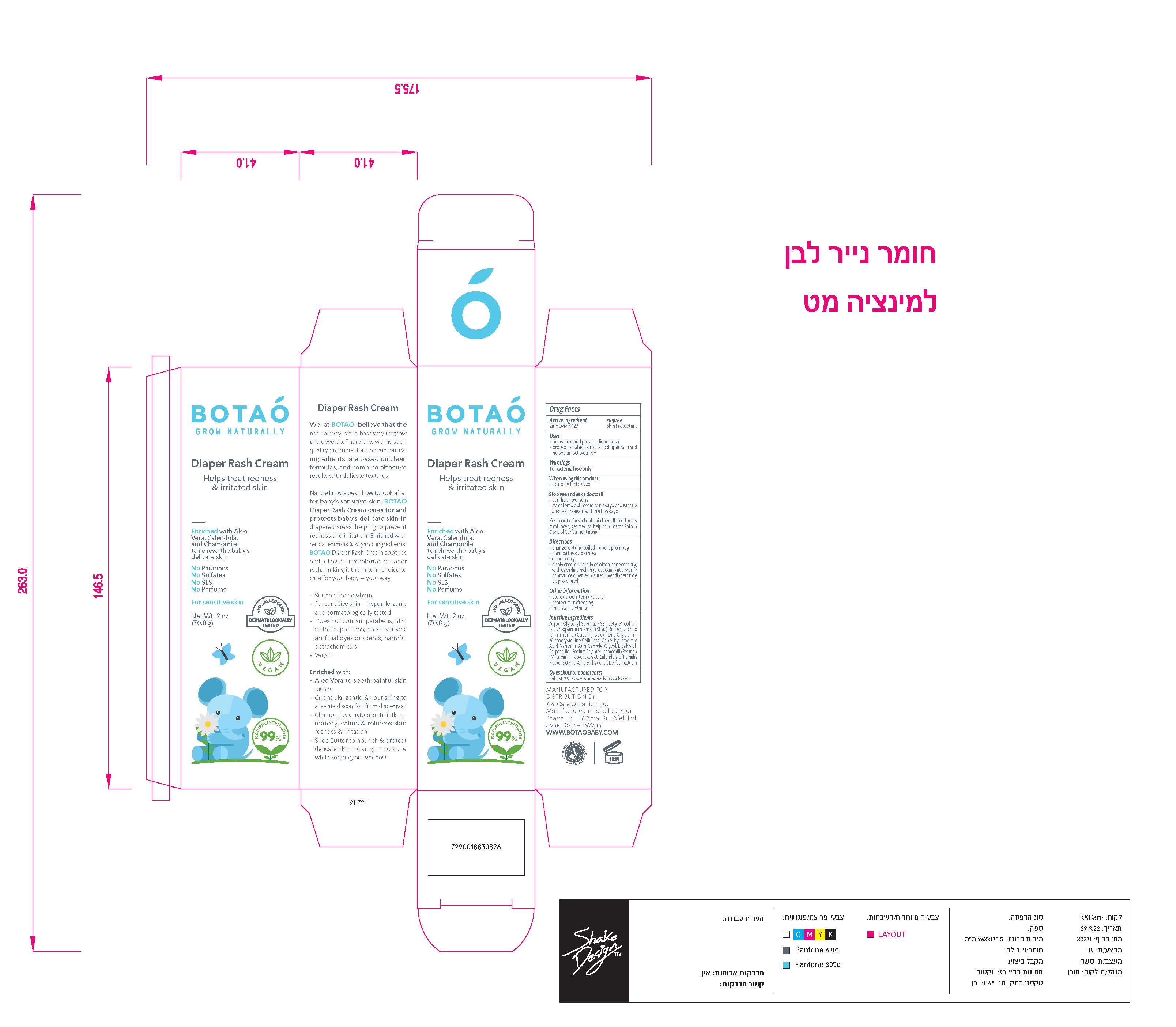
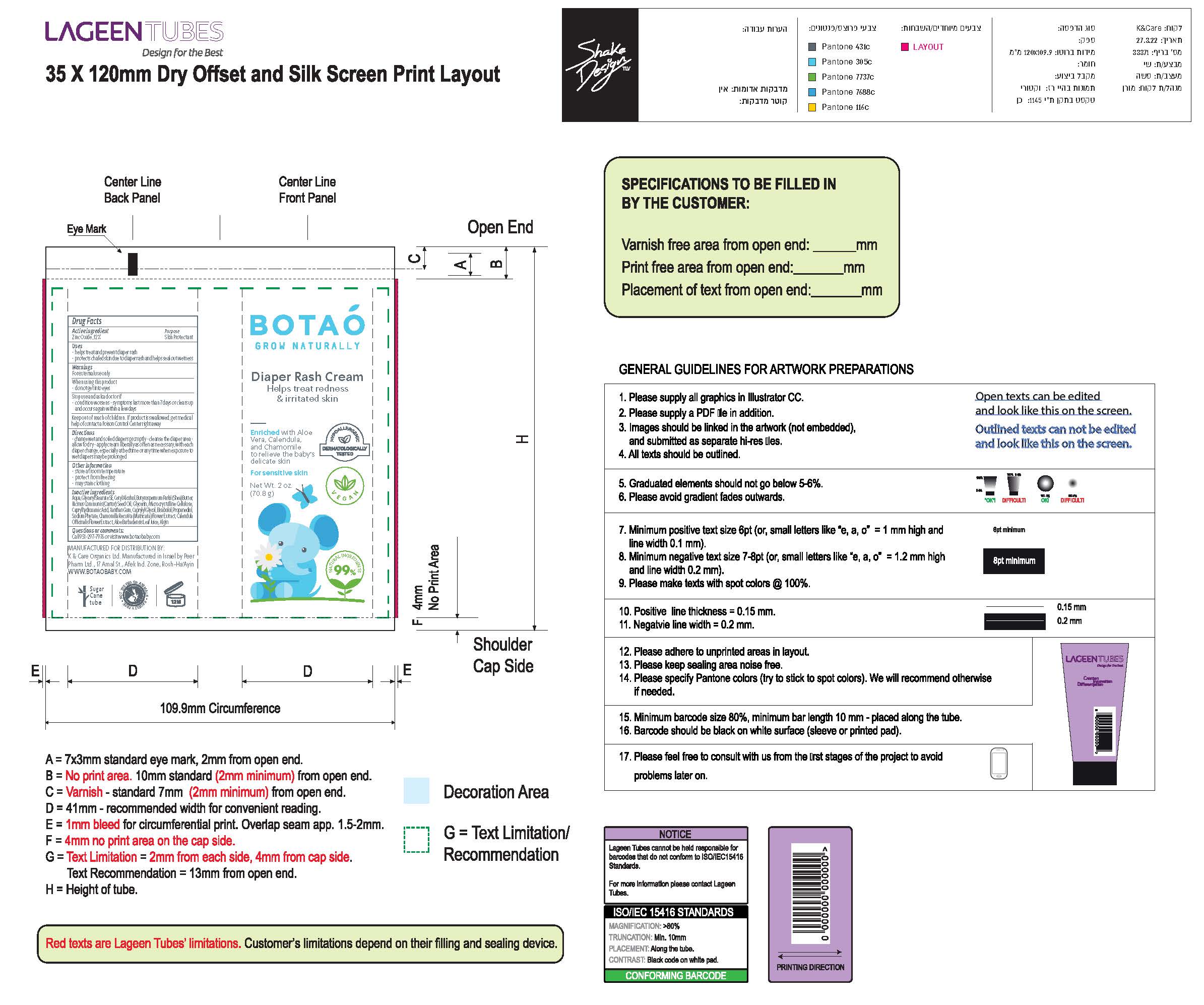
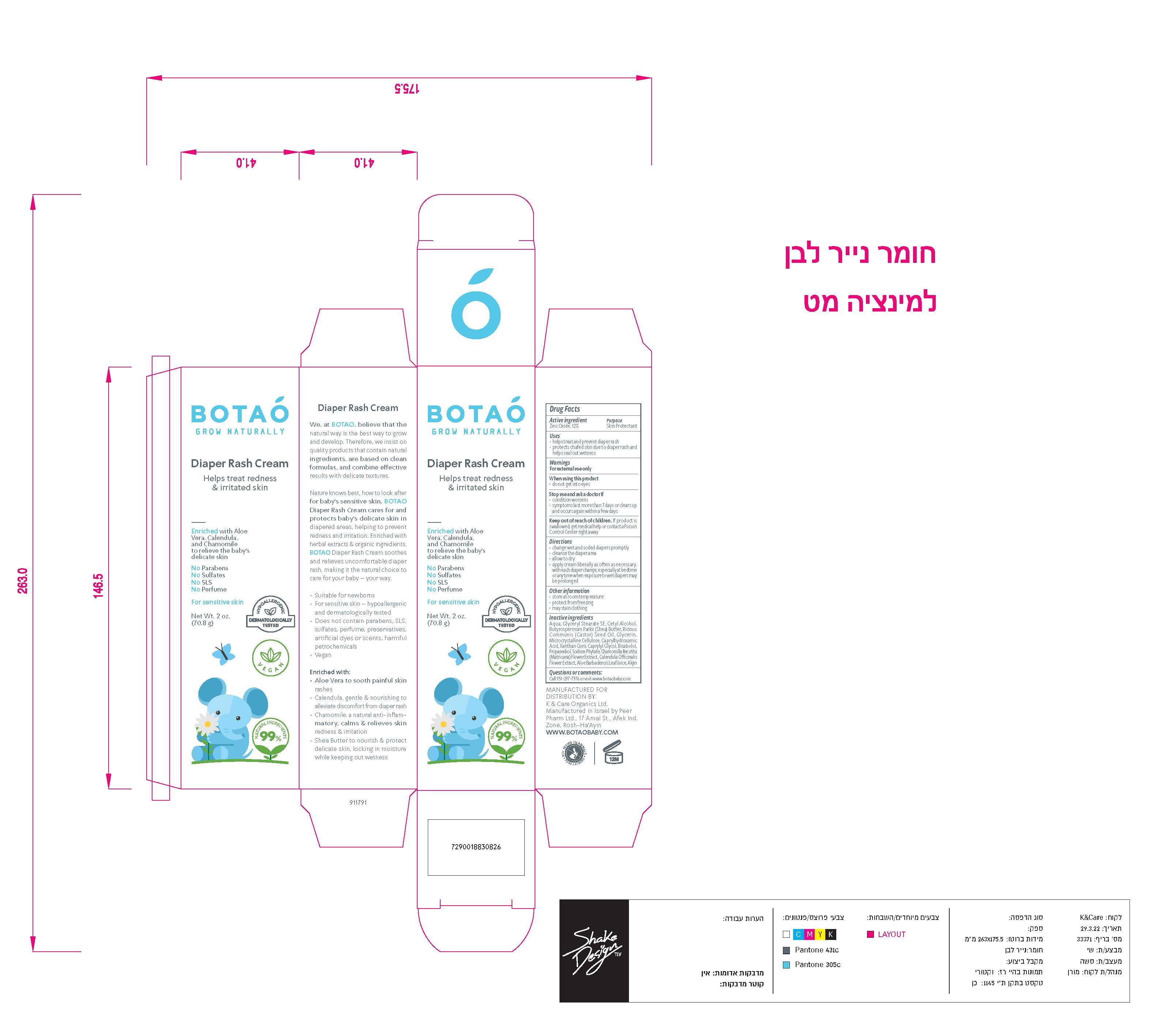
Label: BOTAO BABY DIAPER RASH CREAM- zinc oxide cream
- NDC Code(s): 69435-1501-1, 69435-1501-2
- Packager: Peer Pharm Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 31, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients(in each 30 ml)
- Uses
- warnings
- Directions
-
Inactive Ingredients
Coconut Alkanes, Cocos Nucifera (Virgin Coconut) Oil, Caprylic/Capric Triglyceride, Aloe Barbadensis Leaf Juice, Ricinus Communis (Castor) Seed Oil*, Ethyl Macadamiate, Shea Butter Ethyl Ester, Glycerin*, Polyglyceryl Esters, Polyhydroxystearic Acid, Citrus Aurantium Dulcis (Orange) Peel Wax, Simmondsia Chinensis (Jojoba) Seed Oil*, Jojoba Esters, Coco-Caprylate/Caprate, Copernicia Cerifera (Carnauba) Wax*, Butyrospermum Parkii (Shea Butter) Fruit*, Tocopherol (Vitamin E), Capric Acid, Silica, Disteardimonium Hectorite, Kaolin, Argania Spinosa (Argan) Kernel Oil, Rosa Canina Seed (Rosehip) Oil, Lavandula Angustifolia (Lavender) Oil, Olea Europaea (Olive) Oil, Origanum Vulgare (Oregano) Leaf Extract,
Tocopheryl Acetate, Basabolol, Malic Acid. May Contain: Iron Oxides.
*Certified Organic - Other Information
- Package Labeling
-
INGREDIENTS AND APPEARANCE
BOTAO BABY DIAPER RASH CREAM
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69435-1501 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 9 g in 71 g Inactive Ingredients Ingredient Name Strength HEXASODIUM PHYTATE (UNII: ZBX50UG81V) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALGINIC ACID (UNII: 8C3Z4148WZ) ALOE (UNII: V5VD430YW9) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) WATER (UNII: 059QF0KO0R) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) CETYL ALCOHOL (UNII: 936JST6JCN) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) BUTYROSPERMUM PARKII (SHEA) BUTTER UNSAPONIFIABLES (UNII: 0C9AC7D6XU) RICINUS COMMUNIS SEED (UNII: 7EK4SFN1TX) CASTOR OIL (UNII: D5340Y2I9G) GLYCERIN (UNII: PDC6A3C0OX) PROPANEDIOL 1-PHOSPHATE (UNII: 48G71659RB) CHAMOMILE (UNII: FGL3685T2X) .BETA.-BISABOLOL (UNII: LP618AV2EA) XANTHAN GUM (UNII: TTV12P4NEE) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69435-1501-2 1 in 1 BOX 05/19/2022 1 NDC:69435-1501-1 70.8 g in 1 TUBE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 05/19/2022 Labeler - Peer Pharm Ltd. (514678390) Registrant - Peer Pharm Ltd. (514678390) Establishment Name Address ID/FEI Business Operations Peer Pharm Ltd. 514678390 manufacture(69435-1501)