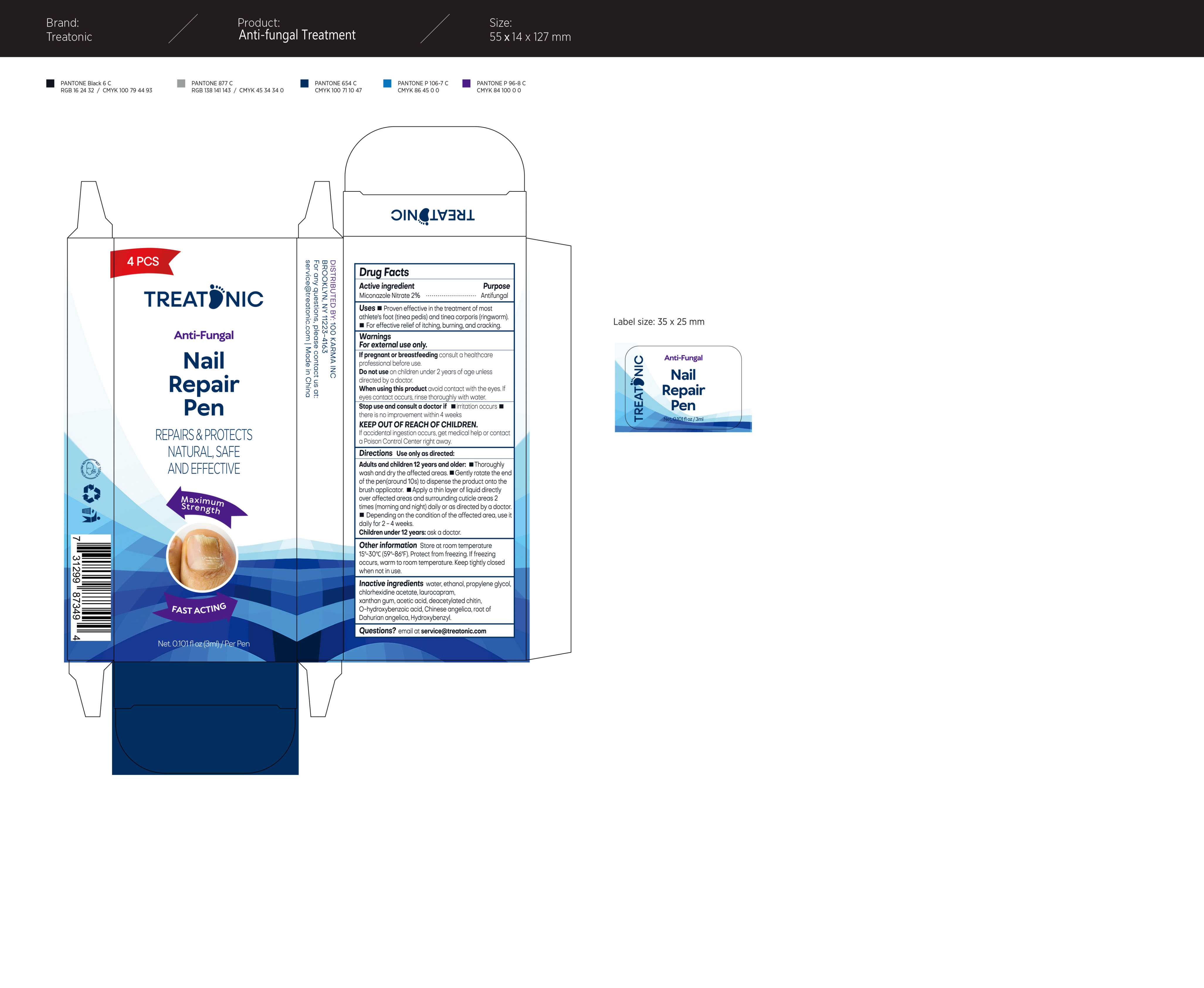
Label: TREATONIC NAIL FUNGUS TREATMENT- nail fungus treatment ointment
- NDC Code(s): 82739-001-01
- Packager: Shenzhen Situya Trading Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated August 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use
- KEEP OUT OF
-
Directions
Directions Use only as directedAdults and children 12 years and older:
Thoroughlyash and dry the affected areas
Gently rotate the endof the pen (around 10s) to dispense the product onto thebrush applicator.
Apply a thin layer of liquid directlyover affected areas and surrounding cuticle areas 2times (morning and night) daily or as directed by a doctor.
Depending on the condition of the affected area , use itdaily for 2-4 weeks
Children under 12 years: ask a doctor
- Other information
- Inactive ingredients
- Package Label-principal Display Panel
-
INGREDIENTS AND APPEARANCE
TREATONIC NAIL FUNGUS TREATMENT
nail fungus treatment ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82739-001 Route of Administration EXTRACORPOREAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MICONAZOLE NITRATE (UNII: VW4H1CYW1K) (MICONAZOLE - UNII:7NNO0D7S5M) MICONAZOLE NITRATE 2 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) CHLORHEXIDINE ACETATE (UNII: 5908ZUF22Y) LAUROCAPRAM (UNII: 1F3X9DRV9X) CHITIN (UNII: 8SH93A7QWW) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) XANTHAN GUM (UNII: TTV12P4NEE) 4-HYDROXYBENZYL ALCOHOL (UNII: 1A3AH1FP1B) ACETIC ACID (UNII: Q40Q9N063P) ANGELICA SINENSIS ROOT (UNII: B66F4574UG) ANGELICA DAHURICA LEAF (UNII: ONF5ZKM88G) SALICYLIC ACID (UNII: O414PZ4LPZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82739-001-01 3 mL in 1 BOX; Type 1: Convenience Kit of Co-Package 05/19/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/19/2022 Labeler - Shenzhen Situya Trading Co., Ltd. (706154255) Establishment Name Address ID/FEI Business Operations Shenzhen Situya Trading Co., Ltd. 706154255 manufacture(82739-001)