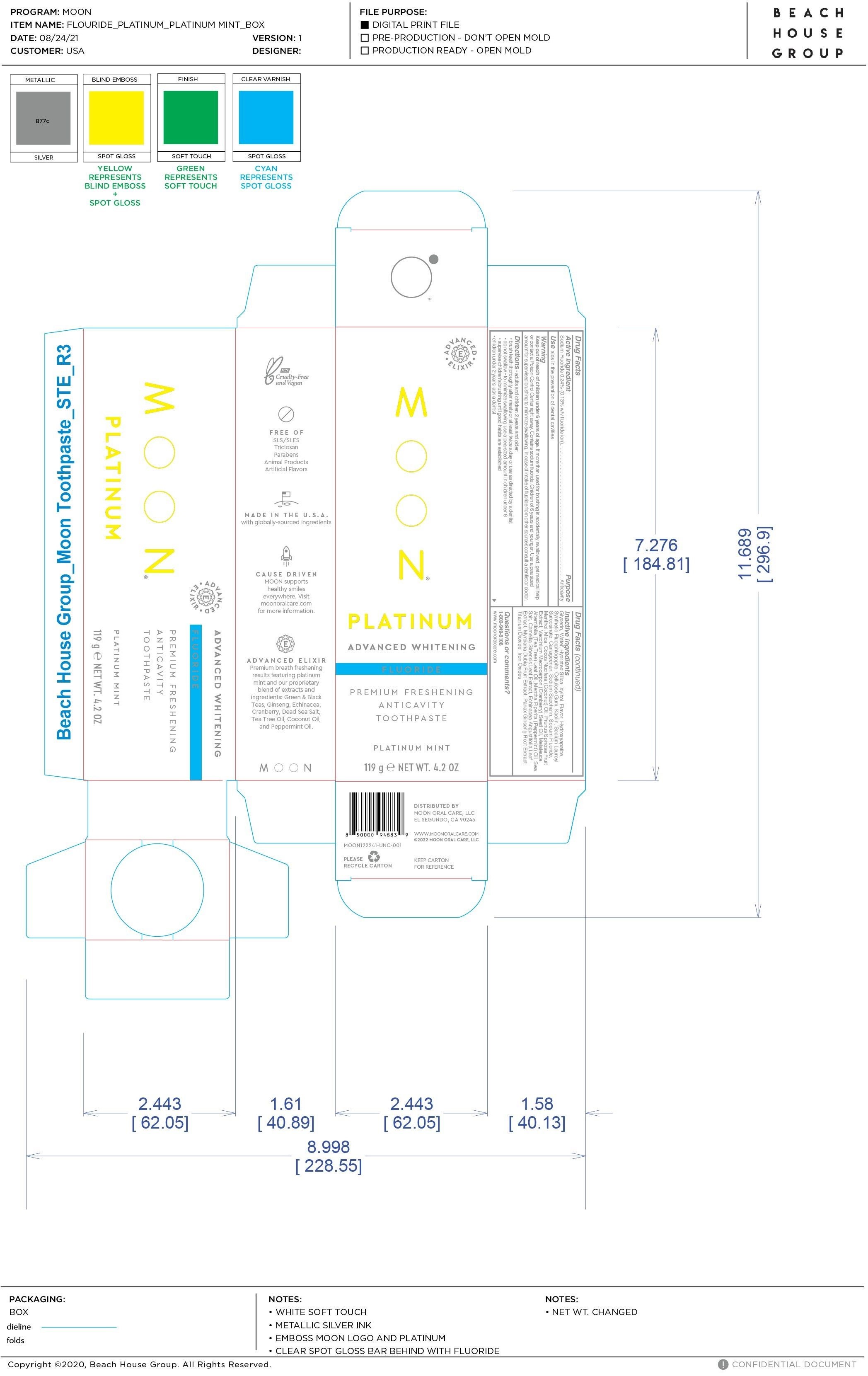
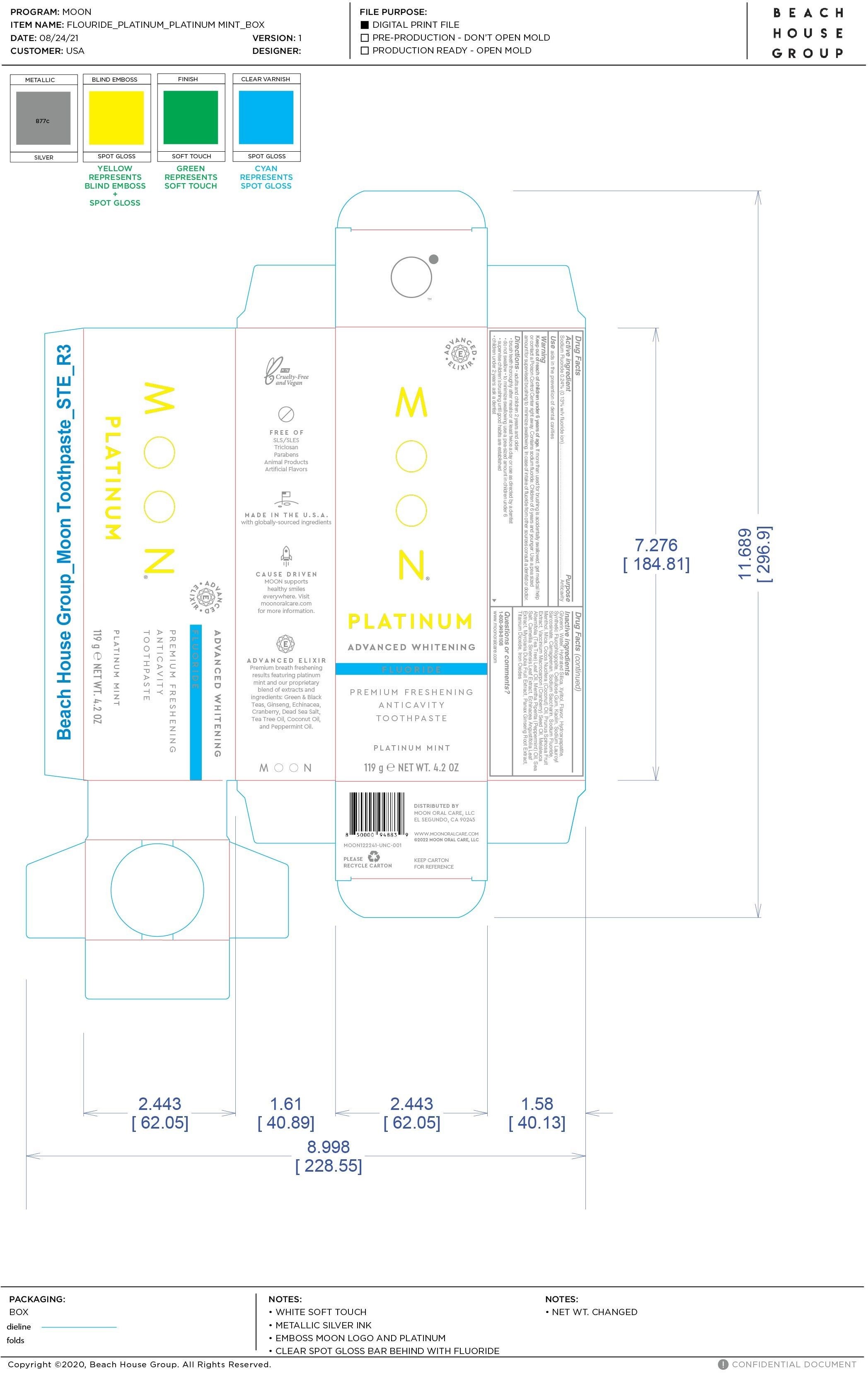
Label: MOON PLATINUM ADVANCED WHITENING ANTICAVITY- sodium fluoride paste
- NDC Code(s): 82214-001-01, 82214-001-02
- Packager: Moon Oral Care LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
Directions
adults and children 2 years and older:
- brush teeth thoroughly after meals at least twice a day or use as directed by a dentist
- do not swallow
- to minimize swallowing, use a pea-sized amount in children under 6
- supervise children's brushing until good habits are established
- children under 2 years ask a dentist
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MOON PLATINUM ADVANCED WHITENING ANTICAVITY
sodium fluoride pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82214-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.24 g in 100 g Inactive Ingredients Ingredient Name Strength TEA TREE OIL (UNII: VIF565UC2G) PEPPERMINT OIL (UNII: AV092KU4JH) WATER (UNII: 059QF0KO0R) CARRAGEENAN (UNII: 5C69YCD2YJ) TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) XYLITOL (UNII: VCQ006KQ1E) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) MENTHOL (UNII: L7T10EIP3A) MICA (UNII: V8A1AW0880) GLYCERIN (UNII: PDC6A3C0OX) HYDRATED SILICA (UNII: Y6O7T4G8P9) SLOE (UNII: 3MLB4858X7) KAOLIN (UNII: 24H4NWX5CO) SODIUM LAUROYL SARCOSINATE (UNII: 632GS99618) MYRCIARIA DUBIA FRUIT (UNII: YSW4EM1EKP) ASIAN GINSENG (UNII: CUQ3A77YXI) SACCHARIN SODIUM (UNII: SB8ZUX40TY) COCONUT OIL (UNII: Q9L0O73W7L) CRANBERRY SEED OIL (UNII: 73KDS3BW5E) GREEN TEA LEAF (UNII: W2ZU1RY8B0) FERRIC OXIDE RED (UNII: 1K09F3G675) SEA SALT (UNII: 87GE52P74G) ECHINACEA ANGUSTIFOLIA LEAF (UNII: FS7G8S6PJ8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82214-001-02 1 in 1 CARTON 01/25/2022 1 NDC:82214-001-01 119 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M022 01/25/2022 Labeler - Moon Oral Care LLC (018021163)