Label: HAIRFX HAIR GROWTH FOAM- minoxidil aerosol, foam
- NDC Code(s): 81653-003-01
- Packager: Guangzhou Jianyuan Biological Technology.Co.,Ltd
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 19, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
-
PURPOSE
STRONGER THICKER LONGER HAIR
REDUCE HAIR LOSS
RESTORE THINNING HAIR
The Foam is for somepeople who have a general thinning of hair on the top of the scalp(vertex only, asshown below). Not intendedfor frontal baldness or areceding hairline. The Foamhas been shown to regrowhair in some people with thefollowing degrees of thinninghair or hair loss.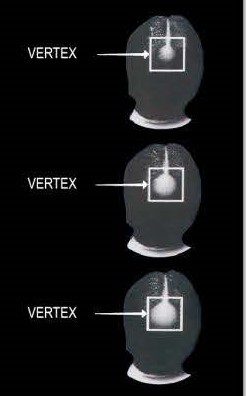
lf you have more hair loss or hair loss in a place different than shown above, Foam may not work. -
lnactive ingredients
Water, Vitamin H (Biotin), Pork Collagen. Panax Ginseng Berry Extract, Carthamus Tinctorius (Safflower) Flower Extract, Frangula Alnus Bark Extract, Ligustrum Lucidum Fruit Extract, Ligusticum Chuanxiong Root Extract, Glycyrrhiza Uralensis (Licorice) Root Extract, Angelica Sinensis Root Extract, Platycladus Orientalis, Polygonum Multiflorum Root Extract, Capsaicin
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- WARNINGS
- WARNINGS AND PRECAUTIONS
-
ROUTE, METHOD AND FREQUENCY OF ADMINISTRATION
USING THE PRODUCT
To open container.
The foam may begin to melt right away on contact with your
warm skin. If your fingers arewarm, rinse them in cold water first.(Be sure to dry them thoroughly before handing thefoam.)
Press nozzle to dispense the topical foam product onto your fingers. The total amount of foam applied should not exceed half a capful.
Using your fingers, spread the foam over the hair loss area
and gently massage into scalp and then wash your hands well. After each use, dose the container to make child resistant by snapping the cap back on to the can. - other information
-
STOP USE
Do not use
- Pregnant women and children.
- on open skin wounds
when using this product
keep out of eyes,ears, and mouth. ln case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor
if irritation or rashoccurs.These may be signs of a serious condition.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison ControlCenter right away. - DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HAIRFX HAIR GROWTH FOAM
minoxidil aerosol, foamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81653-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MINOXIDIL (UNII: 5965120SH1) (MINOXIDIL - UNII:5965120SH1) MINOXIDIL 5 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SAFFLOWER (UNII: 4VBL71TY4Y) LIGUSTICUM SINENSE SUBSP. CHUANXIONG ROOT (UNII: RR83T99U97) LIGUSTRUM LUCIDUM FRUIT (UNII: M9G2U96DUT) ANGELICA SINENSIS ROOT (UNII: B66F4574UG) CAPSAICIN (UNII: S07O44R1ZM) BIOTIN (UNII: 6SO6U10H04) PORK COLLAGEN (UNII: I8442U2G7J) PANAX GINSENG FRUIT (UNII: E35T4MOI3E) FRANGULA ALNUS BARK (UNII: S2D77IH61R) GLYCYRRHIZA URALENSIS ROOT (UNII: 42B5YD8F0K) PLATYCLADUS ORIENTALIS WHOLE (UNII: 835370K47L) REYNOUTRIA MULTIFLORA ROOT (UNII: AUZ3VD75MC) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81653-003-01 60 g in 1 BOTTLE; Type 0: Not a Combination Product 03/27/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/23/2022 Labeler - Guangzhou Jianyuan Biological Technology.Co.,Ltd (548120189) Establishment Name Address ID/FEI Business Operations Guangzhou Jianyuan Biological Technology.Co.,Ltd 548120189 manufacture(81653-003)


