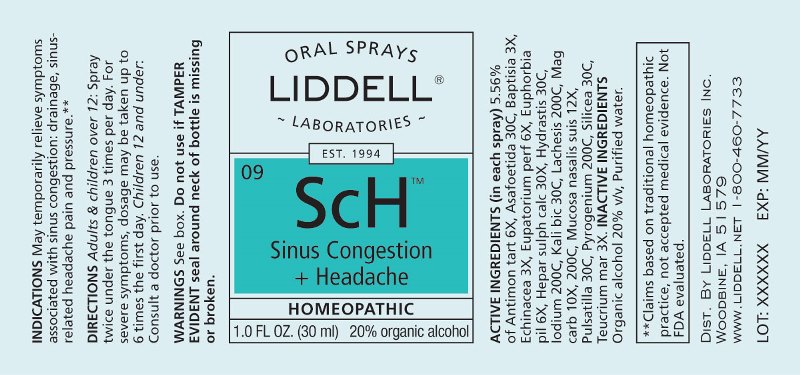
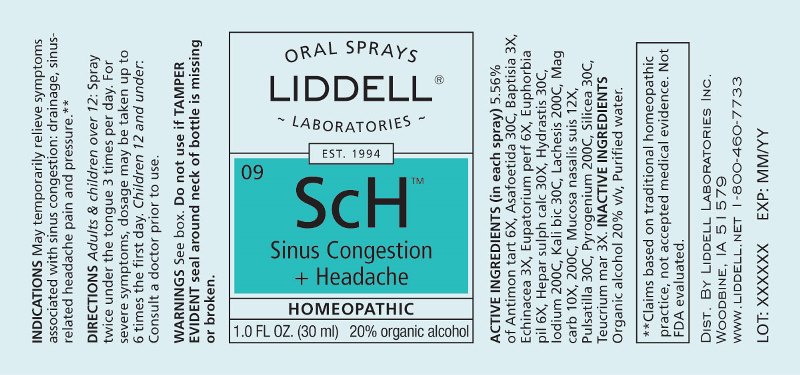
Label: SINUS CONGESTION HEADACHE (antimonium tartaricum, asafoetida, baptisia tinctoria, echinacea (angustifolia), eupatorium perfoliatum, euphorbia pilulifera, hepar sulphuris calcareum, hydrastis canadensis, iodium, kali bichromicum, lachesis mutus, magnesia carbonica, mucosa nasalis suis, pulsatilla- pratensis, pyrogenium, silicea, teucrium marum spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 50845-0263-1, 50845-0263-2 - Packager: Liddell Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated April 22, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
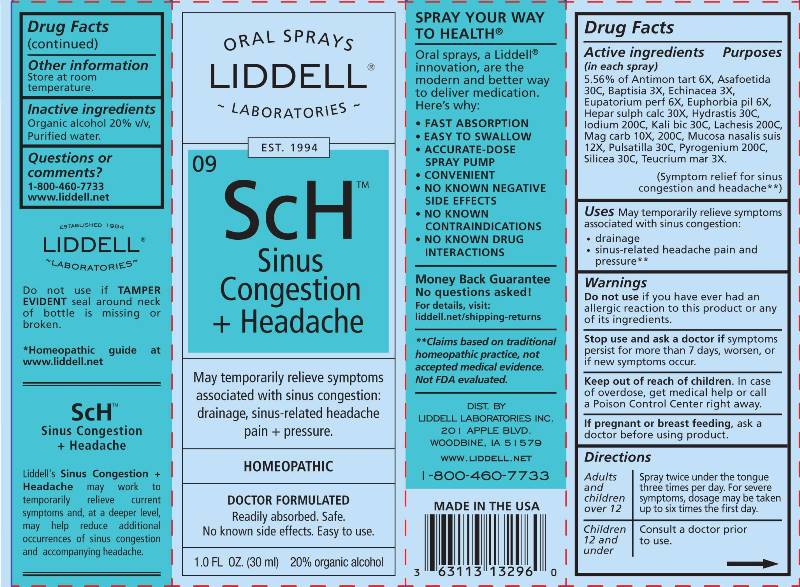
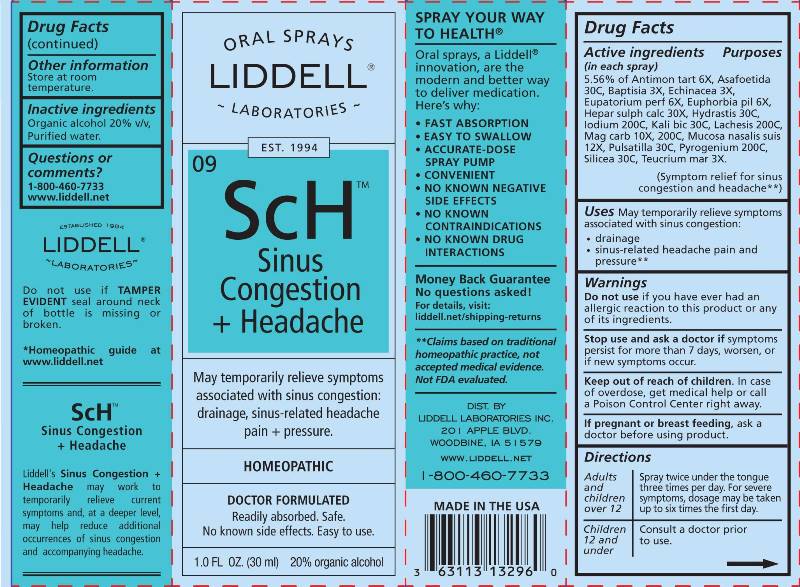
ACTIVE INGREDIENTS:
(in each spray) 5.56% of Antimonium Tartaricum 6X, Asafoetida 30C, Baptisia Tinctoria 3X, Echinacea (Angustifolia) 3X, Eupatorium Perfoliatum 6X, Euphorbia Pilulifera 6X, Hepar Sulphuris Calcareum 30X, Hydrastis Canadensis 30C, Iodium 200C, Kali Bichromicum 30C, Lachesis Mutus 200C, Magnesia Carbonica 10X, 200C, Mucosa Nasalis Suis 12X, Pulsatilla (Pratensis) 30C, Pyrogenium 200C, Silicea 30C, Teucrium Marum 3X.
- USES:
-
WARNINGS:
Do not use if you have ever had an allergic reaction to this product or any of its ingredients.
Stop use and ask a doctor if symptoms persist for more than 7 days, worsen or if new symptoms occur.
Keep out of reach of children. In case of overdose, get medical help or call a Poison Control Center right away.
If pregnant or breast feeding, ask a doctor before using this product.
Do not use if TAMPER EVIDENT seal around neck of bottle is missing or broken.
Store at room temperature.
- KEEP OUT OF REACH OF CHILDREN:
- DIRECTIONS:
- INDICATIONS:
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
SINUS CONGESTION HEADACHE
antimonium tartaricum, asafoetida, baptisia tinctoria, echinacea (angustifolia), eupatorium perfoliatum, euphorbia pilulifera, hepar sulphuris calcareum, hydrastis canadensis, iodium, kali bichromicum, lachesis mutus, magnesia carbonica, mucosa nasalis suis, pulsatilla (pratensis), pyrogenium, silicea, teucrium marum sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50845-0263 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIMONY POTASSIUM TARTRATE (UNII: DL6OZ476V3) (ANTIMONY CATION (3+) - UNII:069647RPT5) ANTIMONY POTASSIUM TARTRATE 6 [hp_X] in 1 mL FERULA ASSA-FOETIDA RESIN (UNII: W9FZA51AS1) (ASAFETIDA - UNII:W9FZA51AS1) FERULA ASSA-FOETIDA RESIN 30 [hp_C] in 1 mL BAPTISIA TINCTORIA ROOT (UNII: 5EF0HWI5WU) (BAPTISIA TINCTORIA ROOT - UNII:5EF0HWI5WU) BAPTISIA TINCTORIA ROOT 3 [hp_X] in 1 mL ECHINACEA ANGUSTIFOLIA WHOLE (UNII: VB06AV5US8) (ECHINACEA ANGUSTIFOLIA - UNII:VB06AV5US8) ECHINACEA ANGUSTIFOLIA WHOLE 3 [hp_X] in 1 mL EUPATORIUM PERFOLIATUM FLOWERING TOP (UNII: 1W0775VX6E) (EUPATORIUM PERFOLIATUM FLOWERING TOP - UNII:1W0775VX6E) EUPATORIUM PERFOLIATUM FLOWERING TOP 6 [hp_X] in 1 mL EUPHORBIA HIRTA FLOWERING TOP (UNII: 6H89ZY31MR) (EUPHORBIA HIRTA FLOWERING TOP - UNII:6H89ZY31MR) EUPHORBIA HIRTA FLOWERING TOP 6 [hp_X] in 1 mL CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 30 [hp_X] in 1 mL GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 30 [hp_C] in 1 mL IODINE (UNII: 9679TC07X4) (IODINE - UNII:9679TC07X4) IODINE 200 [hp_C] in 1 mL POTASSIUM DICHROMATE (UNII: T4423S18FM) (DICHROMATE ION - UNII:9LKY4BFN2V) POTASSIUM DICHROMATE 30 [hp_X] in 1 mL LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 200 [hp_C] in 1 mL MAGNESIUM CARBONATE (UNII: 0E53J927NA) (CARBONATE ION - UNII:7UJQ5OPE7D) MAGNESIUM CARBONATE 10 [hp_X] in 1 mL SUS SCROFA NASAL MUCOSA (UNII: ID3Z1X61WY) (SUS SCROFA NASAL MUCOSA - UNII:ID3Z1X61WY) SUS SCROFA NASAL MUCOSA 12 [hp_X] in 1 mL PULSATILLA PRATENSIS WHOLE (UNII: 8E272251DI) (PULSATILLA PRATENSIS WHOLE - UNII:8E272251DI) PULSATILLA PRATENSIS WHOLE 30 [hp_C] in 1 mL RANCID BEEF (UNII: 29SUH5R3HU) (RANCID BEEF - UNII:29SUH5R3HU) RANCID BEEF 200 [hp_C] in 1 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 30 [hp_C] in 1 mL TEUCRIUM MARUM WHOLE (UNII: 10464S0TAA) (TEUCRIUM MARUM - UNII:10464S0TAA) TEUCRIUM MARUM WHOLE 3 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50845-0263-2 1 in 1 PACKAGE 04/24/2020 1 NDC:50845-0263-1 30 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 04/24/2020 Labeler - Liddell Laboratories, Inc. (832264241) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(50845-0263) , api manufacture(50845-0263) , label(50845-0263) , pack(50845-0263)