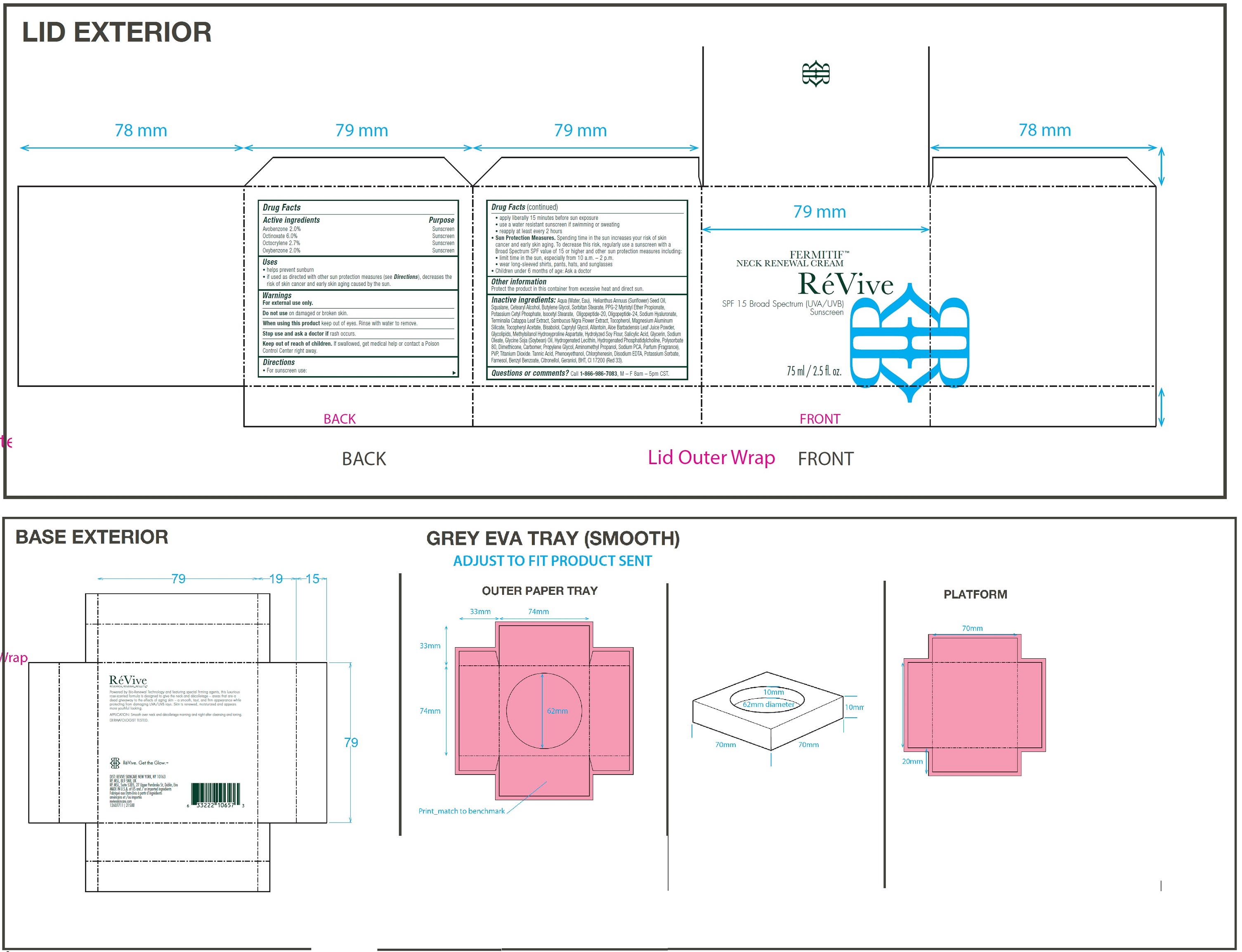
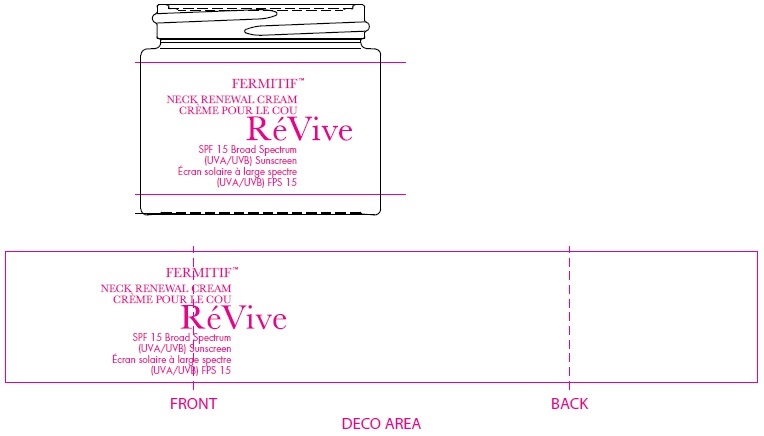
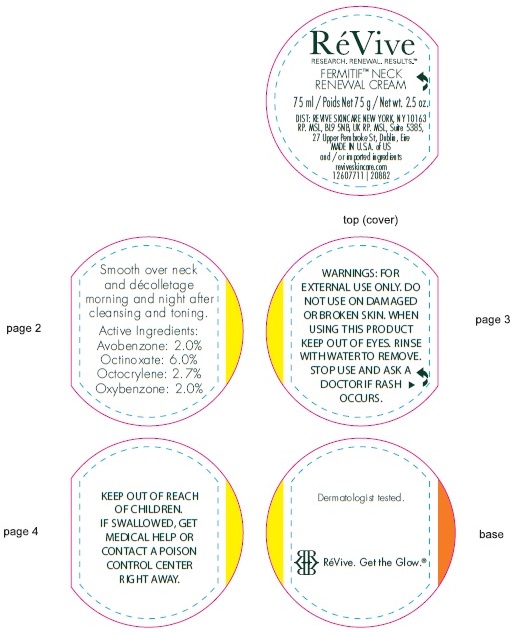
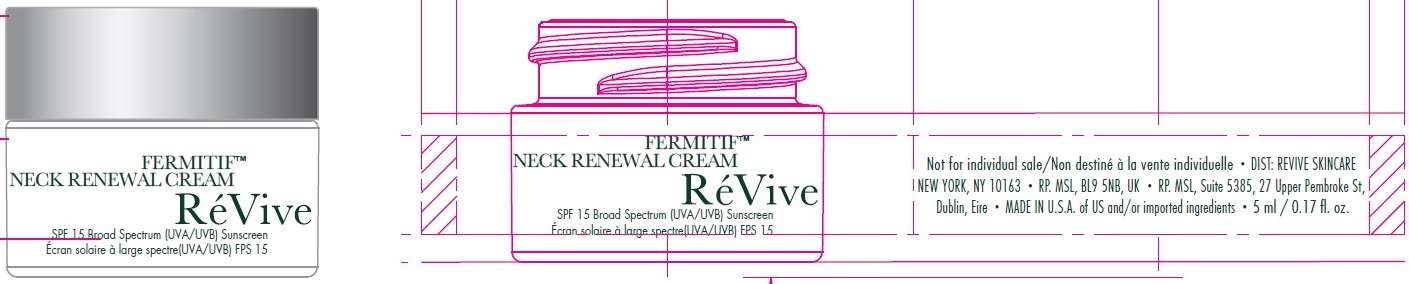
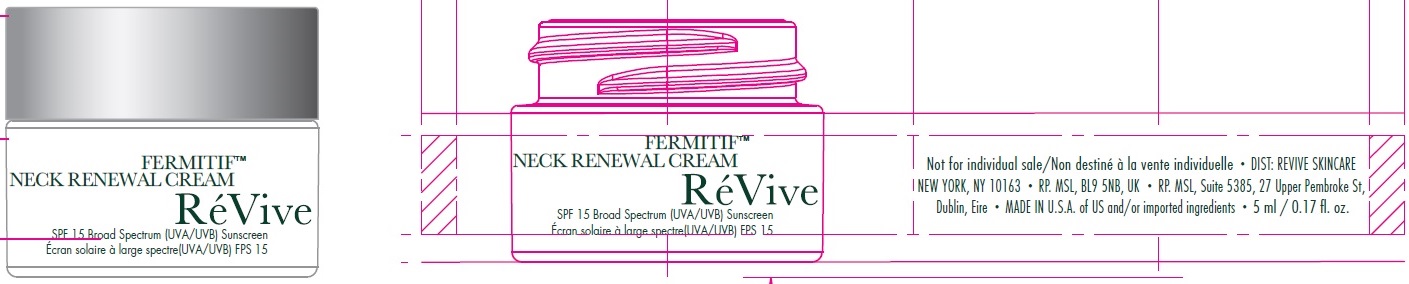
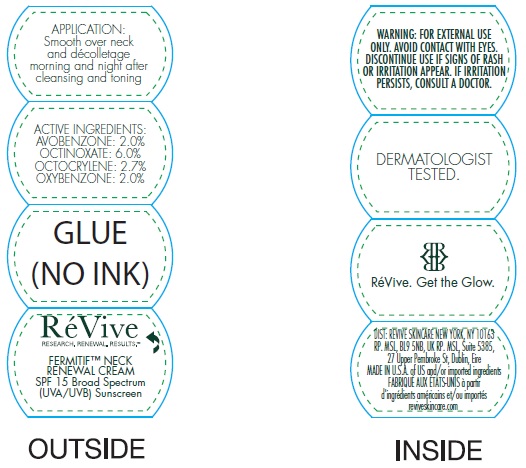
Label: FERMITIF NECK RENEWAL CREAM BROAD SPECTRUM SPF 15 SUNSCREEN US- avobenzone, octinoxate, octocrylene, oxybenzone cream
- NDC Code(s): 82691-141-00, 82691-141-01
- Packager: RV Skincare LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
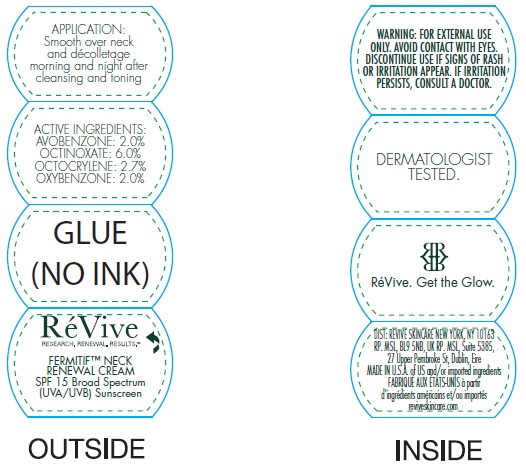
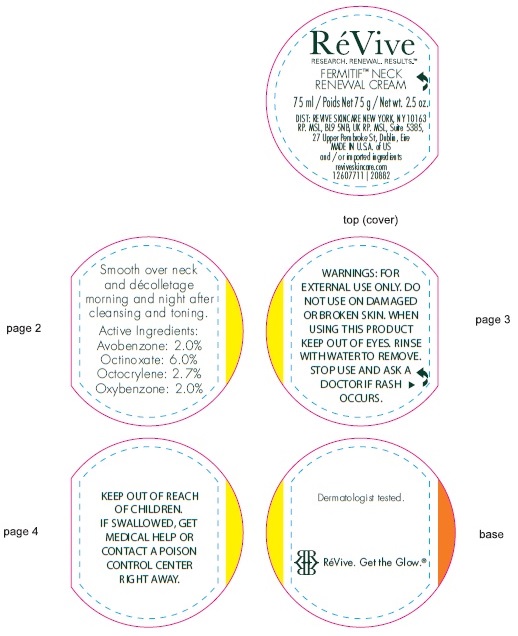
- Active ingredients
- Uses
- Warnings
-
Directions
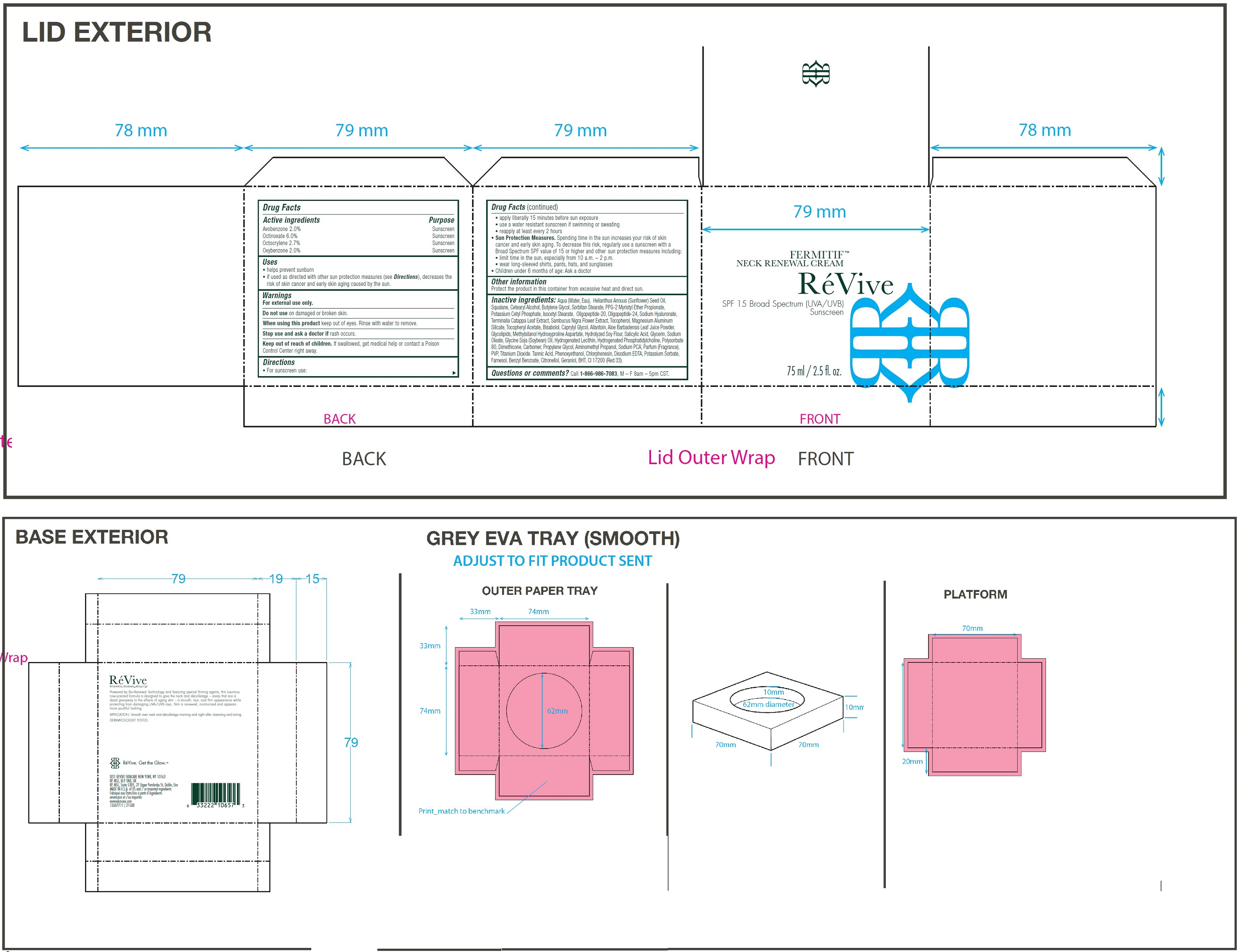
• For sunscreen use: • apply liberally 15 minutes before sun exposure • use a water resistant sunscreen if swimming or sweating • reapply at least every 2 hours • Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: • limit time in the sun, especially from 10 a.m. – 2 p.m. • wear long-sleeved shirts, pants, hats, and sunglasses • Children under 6 months of age: Ask a doctor
Sun Protection Measures. - Other information
-
Inactive ingredients:
Aqua (Water, Eau), Helianthus Annuus (Sunflower) Seed Oil, Squalane, Cetearyl Alcohol, Butylene Glycol, Sorbitan Stearate, PPG-2 Myristyl Ether Propionate, Potassium Cetyl Phosphate, Isocetyl Stearate, Oligopeptide-20, Oligopeptide-24, Sodium Hyaluronate, Terminalia Catappa Leaf Extract, Sambucus Nigra Flower Extract, Tocopherol, Magnesium Aluminum Silicate, Tocopheryl Acetate, Bisabolol, Caprylyl Glycol, Allantoin, Aloe Barbadensis Leaf Juice Powder, Glycolipids, Methylsilanol Hydroxyproline Aspartate, Hydrolyzed Soy Flour, Salicylic Acid, Glycerin, Sodium Oleate, Glycine Soja (Soybean) Oil, Hydrogenated Lecithin, Hydrogenated Phosphatidylcholine, Polysorbate 80, Dimethicone, Carbomer, Propylene Glycol, Aminomethyl Propanol, Sodium PCA, Parfum (Fragrance), PVP, Titanium Dioxide. Tannic Acid, Phenoxyethanol, Chlorphenesin, Disodium EDTA, Potassium Sorbate, Farnesol, Benzyl Benzoate, Citronellol, Geraniol, BHT, CI 17200 (Red 33).
- Questions or comments?
- Package Labeling:
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
FERMITIF NECK RENEWAL CREAM BROAD SPECTRUM SPF 15 SUNSCREEN US
avobenzone, octinoxate, octocrylene, oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82691-141 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 20 mg in 1 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 60 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 27 mg in 1 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SUNFLOWER OIL (UNII: 3W1JG795YI) SQUALANE (UNII: GW89575KF9) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) PPG-2 MYRISTYL ETHER PROPIONATE (UNII: 88R97D8U8A) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) ISOCETYL STEARATE (UNII: 3RJ7186O9W) HYALURONATE SODIUM (UNII: YSE9PPT4TH) TERMINALIA CATAPPA LEAF (UNII: 4XBZ9J585L) SAMBUCUS NIGRA FLOWER (UNII: 07V4DX094T) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) LEVOMENOL (UNII: 24WE03BX2T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALLANTOIN (UNII: 344S277G0Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) SALICYLIC ACID (UNII: O414PZ4LPZ) GLYCERIN (UNII: PDC6A3C0OX) SODIUM OLEATE (UNII: 399SL044HN) SOYBEAN OIL (UNII: 241ATL177A) POLYSORBATE 80 (UNII: 6OZP39ZG8H) DIMETHICONE (UNII: 92RU3N3Y1O) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TANNIC ACID (UNII: 28F9E0DJY6) PHENOXYETHANOL (UNII: HIE492ZZ3T) CHLORPHENESIN (UNII: I670DAL4SZ) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FARNESOL (UNII: EB41QIU6JL) BENZYL BENZOATE (UNII: N863NB338G) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) GERANIOL (UNII: L837108USY) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) D&C RED NO. 33 (UNII: 9DBA0SBB0L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82691-141-00 1 in 1 CARTON 12/01/2017 1 75 mL in 1 JAR; Type 0: Not a Combination Product 2 NDC:82691-141-01 5 mL in 1 JAR; Type 0: Not a Combination Product 12/01/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/01/2017 Labeler - RV Skincare LLC (080986653)