Label: AXIM ALLERGY- diphenhydramine hydrochloride capsule, liquid filled
- NDC Code(s): 82706-005-01, 82706-005-02
- Packager: VIVUNT LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
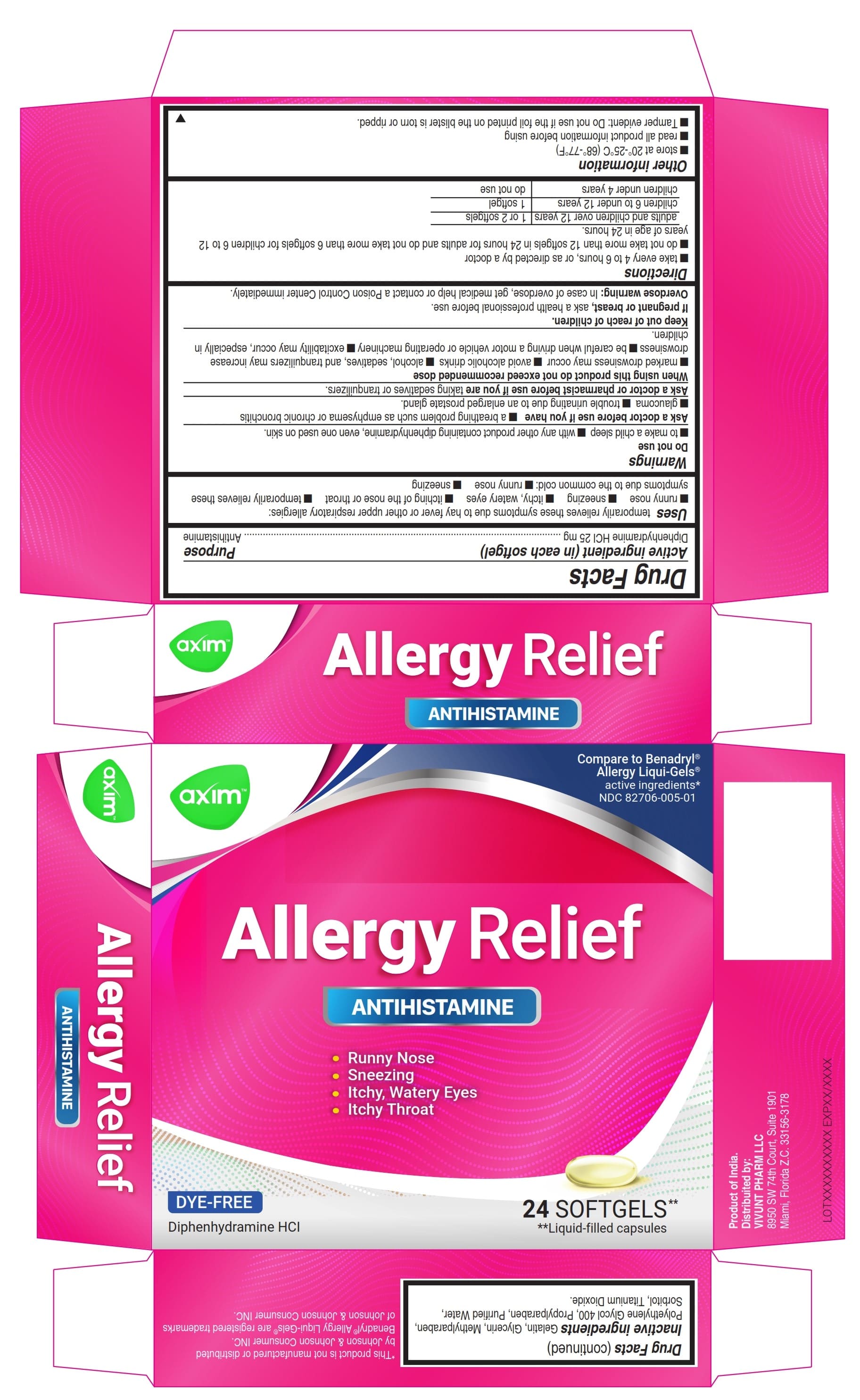
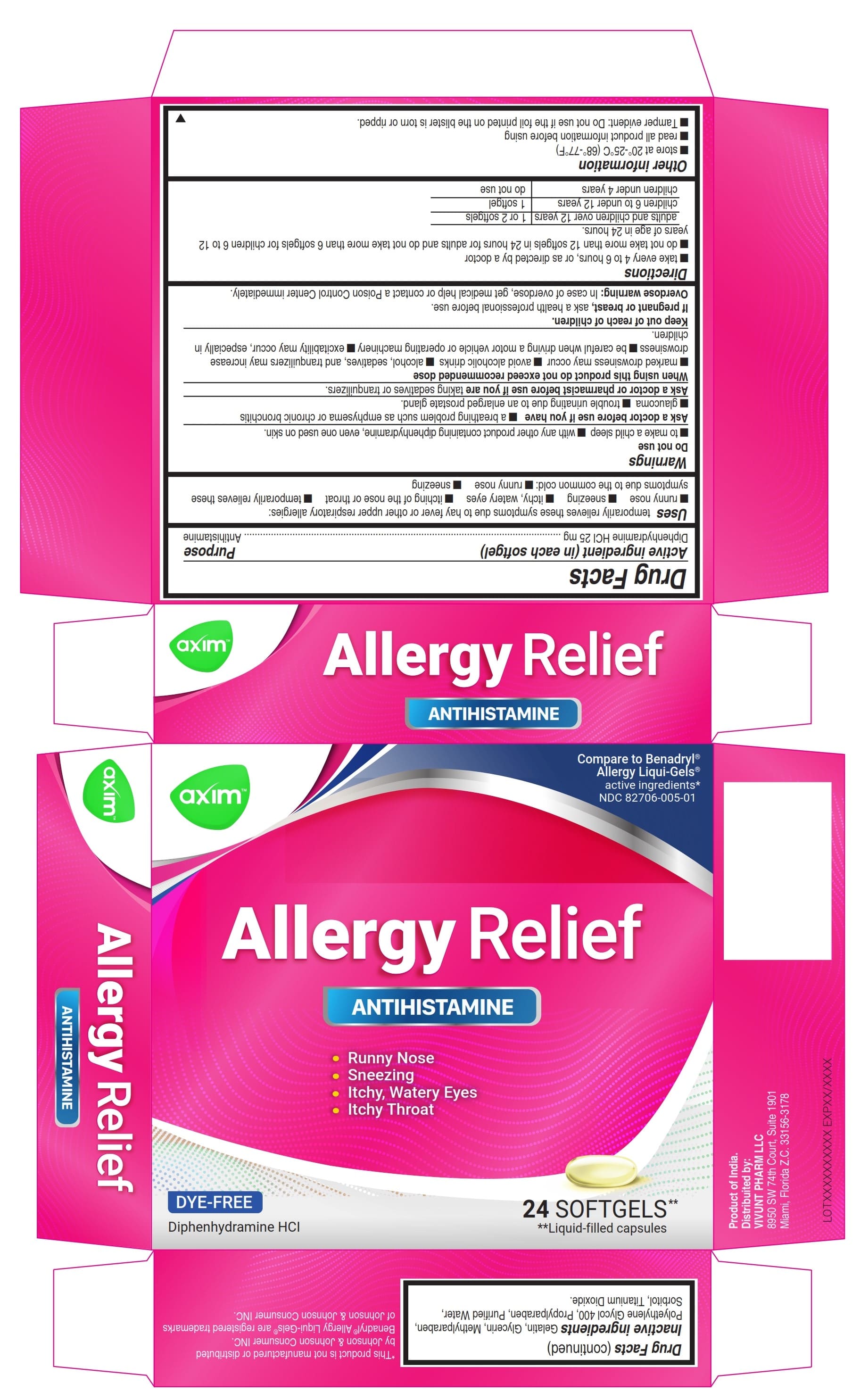
- ACTIVE INGREDIENT
- Uses
-
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
-
Directions
- Take every 4 to 6 hours, or as directed by a doctor
- Do not take more than 12 Softgels in 24 hours for Adults and do not take more than 6 Softgels for children 6 to 12 years of age in 24 hours.
adults and children 12 years and over 1 or 2 Softgels children 6 to under 12 years 1 Softgel children under 6 years do not use - Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL 24
- PRINCIPAL DISPLAY PANEL 6
-
INGREDIENTS AND APPEARANCE
AXIM ALLERGY
diphenhydramine hydrochloride capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82706-005 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength PROPYLPARABEN (UNII: Z8IX2SC1OH) GELATIN (UNII: 2G86QN327L) METHYLPARABEN (UNII: A2I8C7HI9T) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) SORBITOL (UNII: 506T60A25R) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white (Transparent) Score no score Shape OVAL Size 22mm Flavor Imprint Code axim Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82706-005-01 2 in 1 CARTON 05/09/2022 1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:82706-005-02 3 in 1 CARTON 09/20/2023 09/20/2023 2 2 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 05/09/2022 Labeler - VIVUNT LLC (045829437)