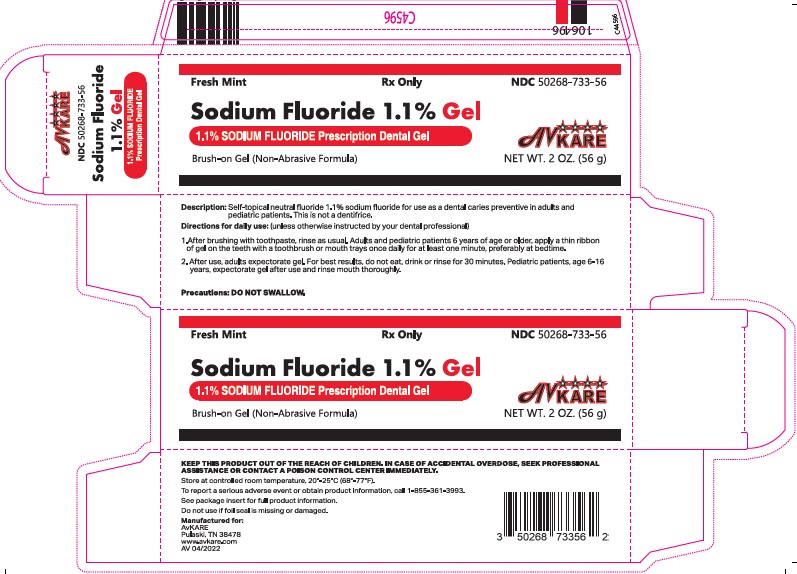
Label: SODIUM FLUORIDE GEL- sodium fluoride gel, dentifrice
- NDC Code(s): 50268-733-56
- Packager: AvPAK
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- DESCRIPTION
- CLINICAL PHARMACOLOGY
-
INDICATIONS AND USAGE
A dental caries preventive, for once daily self-applied topical use. It is well established that 1.1% sodium fluoride is safe and extraordinarily effective as a caries preventive when applied frequently with mouthpiece applicators. Sodium Fluoride 1.1% Gel in a squeeze-tube is easily applied onto a toothbrush as well as a mouthpiece tray. This prescription dental gel should be used once daily following use of a regular toothpaste unless otherwise instructed by your dental professional. May be used whether or not drinking water is fluoridated since topical fluoride cannot produce fluorosis. (See WARNINGS for exception.)
- CONTRAINDICATIONS
-
WARNINGS
Prolonged daily ingestion may result in various degrees of dental fluorosis in pediatric patients under 6 years,
especially if the water fluoridation exceeds 0.6 ppm, since younger pediatric patients frequently cannot perform the
brushing process without significant swallowing. Use in pediatric patients under age 6 years requires special
supervision to prevent repeated swallowing of gel which could cause dental fluorosis. Read directions carefully
before using. If using a mouthpiece application, prolonged exposure (longer than 1 minute) may result in oral
irritation, such as burning. -
PRECAUTIONS
KEEP THIS PRODUCT OUT OF THE REACH OF CHILDREN. IN CASE OF ACCIDENTAL OVERDOSE, SEEK
PROFESSIONAL ASSISTANCE OR CONTACT A POISON CONTROL CENTER IMMEDIATELYCarcinogenesis, Mutagenesis, Impairment of Fertility
In a study conducted in rodents, no carcinogenesis was found in male and female mice and female rats treated with fluoride at dose levels ranging from 4.1 to 9.1 mg/kg of body weight. Equivocal evidence of carcinogenesis was reported in male rats treated with 2.5 and 4.1 mg/kg of body weight. In a second study, no carcinogenesis was observed in rats, males or females, treated with fluoride up to 11.3 mg/kg of body weight. Epidemiological data provide no credible evidence for an association between fluoride, either naturally occurring or added to drinking water, and risk of human cancer.
Fluoride ion is not mutagenic in standard bacterial systems. It has been shown that fluoride ion has potential to induce chromosome aberrations in cultured human and rodent cells at doses much higher than those to which humans are exposed. In vivo data are also conflicting. Some studies report chromosome damage in rodents, while other studies using similar protocols report negative results.
Potential adverse reproductive effects of fluoride exposure in humans has not been adequately evaluated. Adverse effects on reproduction were reported for rats, mice, fox, and cattle exposed to 100 ppm or greater concentrations of fluoride in their diet or drinking water. Other studies conducted in rats demonstrated that lower concentrations of fluoride (5 mg/kg of body weight) did not result in impaired fertility and reproductive capabilities.
Pregnancy
Teratogenic Effects
Pregnancy Category B
It has been shown that fluoride crosses the placenta of rats, but only 0.01% of the amount administered is incorporated in fetal tissue. Animal studies (rats, mice, rabbits) have shown that fluoride is not a teratogen. Maternal exposure to 12.2 mg fluoride/kg of body weight (rats) or 13.1 mg/kg of body weight (rabbits) did not affect the litter size or fetal weight and did not increase the frequency of skeletal or visceral malformations. There are no adequate and well-controlled studies in pregnant women. However, epidemiological studies conducted in areas with high levels of naturally fluoridated water showed no increase in birth defects. Heavy exposure to fluoride during in utero development may result in skeletal fluorosis which becomes evident in childhood.
Nursing Mothers
It is not known if fluoride is excreted in human milk. However, many drugs are excreted in milk, and caution should be exercised when products containing fluoride are administered to a nursing woman. Reduced milk production was reported in farm-raised fox when the animals were fed a diet containing a high concentration of fluoride (98-137 mg/kg of body weight). No adverse effects on parturition, lactation, or offspring were seen in rats administered fluoride up to 5 mg/kg of body weight.
Pediatric Use
The use of PreviDent® Gel in pediatric age groups 6 to 16 years as a caries preventive is supported by pioneering clinical studies with 1.1% sodium fluoride gels in mouth trays in students age 11-14 years conducted by Englander, et al. 2,3,4 Safety and effectiveness in pediatric patients below the age of 6 years have not been established. Please refer to the CONTRAINDICATIONS and WARNINGS sections.
Geriatric Use
Of the total number of subjects in clinical studies of 1.1% (w/v) sodium fluoride, 15 percent were 65 and over, while 1 percent were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
- ADVERSE REACTIONS
-
OVERDOSAGE
Accidental ingestion of large amounts of fluoride may result in acute burning in the mouth and sore tongue. Nausea, vomiting, and diarrhea may occur soon after ingestion (within 30 minutes) and are accompanied by salivation, hematemesis, and epigastric cramping abdominal pain. These symptoms may persist for 24 hours. If less than 5 mg fluoride/kg body weight (i.e., less than 2.3 mg fluoride/lb body weight) have been ingested, give calcium (e.g., milk) orally to relieve gastrointestinal symptoms and observe for a few hours. If more than 5 mg fluoride/kg body weight (i.e., more than 2.3 mg fluoride/lb body weight) have been ingested, induce vomiting, give orally soluble calcium (e.g., milk, 5% calcium gluconate or calcium lactate solution) and immediately seek medical assistance. For accidental ingestion of more than 15 mg fluoride/kg of body weight (i.e., more than 6.9 mg fluoride/lb body weight), induce vomiting and admit immediately to a hospital facility.
A treatment dose (a thin ribbon) of PreviDent® Gel contains approximately 2 mg fluoride. A 2 oz. tube contains approximately 266 mg fluoride.
-
DOSAGE AND ADMINISTRATION
Follow these instructions unless otherwise instructed by your dental professional:
Directions for daily use:
1. After brushing thoroughly with toothpaste, rinse as usual. Adults and pediatric patients 6 years of age or older,
apply a thin ribbon of gel to the teeth with a toothbrush or mouth trays once daily for at least one minute,
preferably at bedtime.
2. After use, adults expectorate gel. For the best results, do not eat, drink, or rinse for 30 minutes. Pediatric
patients, age 6-16 years, expectorate gel after use and rinse mouth thoroughly.Precaution: DO NOT SWALLOW.
- HOW SUPPLIED
-
REFERENCES
1. American Dental Association, Council on Dental Therapeutics, Fluoride compounds, In: Accepted Dental Therapeutics, Ed. 40, Chicago, ADA, 405-407 (1984). 2. H.R. Englander et al., Clinical Anticaries Effect of Repeated Topical Sodium Fluoride Applications by Mouthpieces, JADA, 75, 638-644 (1967). 3. H.R. Englander et al., Residual Anticaries Effect of Repeated Topical Sodium Fluoride Applications by Mouthpieces, JADA, 78, 783-787 (1969). 4. H.R. Englander et al., Incremental Rates of Dental Caries After Repeated Topical Sodium Fluoride Applications in ChildrenWith Lifelong Consumption of FluoridatedWater, JADA 82, 354-358, (1971)
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 56g Tube
-
INGREDIENTS AND APPEARANCE
SODIUM FLUORIDE GEL
sodium fluoride gel, dentifriceProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50268-733 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 5 mg in 1 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) SODIUM BENZOATE (UNII: OJ245FE5EU) XYLITOL (UNII: VCQ006KQ1E) SUCROSE (UNII: C151H8M554) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) HYDROXYETHYL CELLULOSE (2000 MPA.S AT 1%) (UNII: S38J6RZN16) Product Characteristics Color blue (Opaque light blue) Score Shape Size Flavor PEPPERMINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50268-733-56 1 in 1 CARTON 05/09/2022 1 56 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/09/2022 Labeler - AvPAK (832926666)