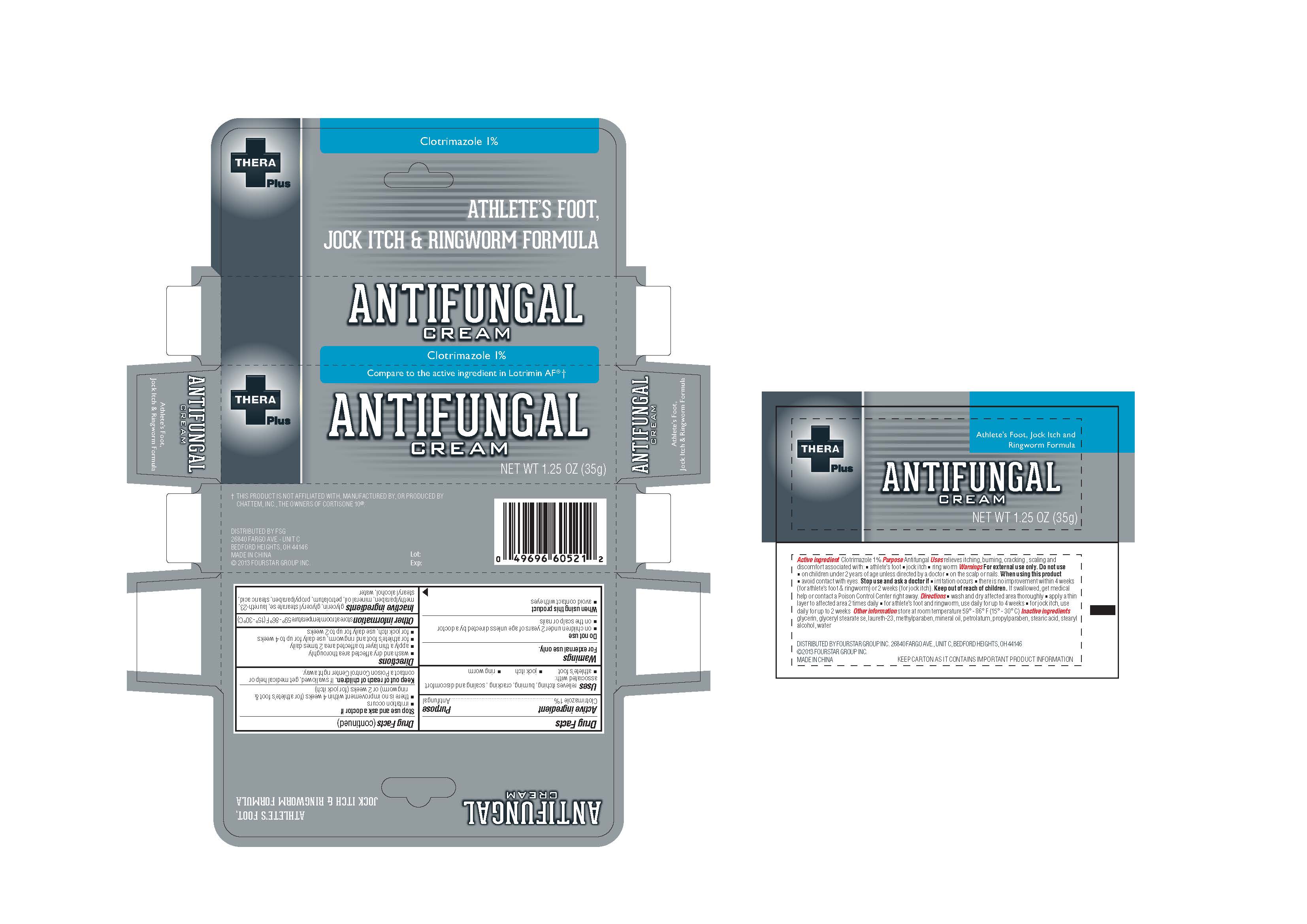
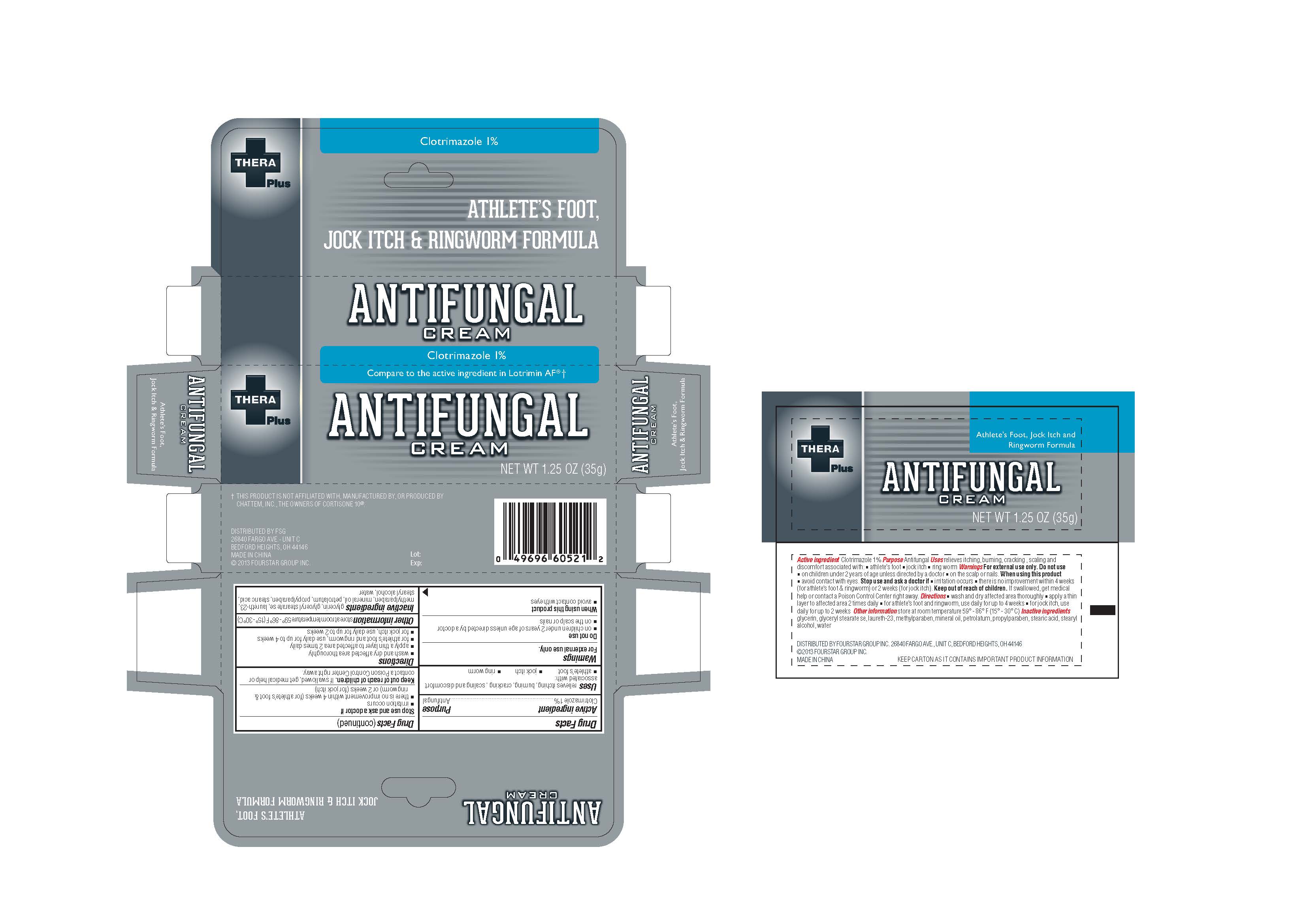
Label: THERAPLUS PLUS- clotrimazole cream
- NDC Code(s): 55621-003-01
- Packager: Zhejiang Jingwei Pharmaceutical Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INACTIVE INGREDIENT
- ACTIVE INGREDIENT
- WARNINGS
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
THERAPLUS PLUS
clotrimazole creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55621-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLOTRIMAZOLE (UNII: G07GZ97H65) (CLOTRIMAZOLE - UNII:G07GZ97H65) CLOTRIMAZOLE 1 g in 100 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) LAURETH-23 (UNII: N72LMW566G) METHYLPARABEN (UNII: A2I8C7HI9T) MINERAL OIL (UNII: T5L8T28FGP) PETROLATUM (UNII: 4T6H12BN9U) PROPYLPARABEN (UNII: Z8IX2SC1OH) STEARIC ACID (UNII: 4ELV7Z65AP) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55621-003-01 35 g in 1 TUBE; Type 0: Not a Combination Product 03/01/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 03/01/2013 Labeler - Zhejiang Jingwei Pharmaceutical Co., Ltd. (530876549) Establishment Name Address ID/FEI Business Operations Zhejiang Jingwei Pharmaceutical Co., Ltd. 530876549 manufacture(55621-003)