Label: REGLAN- metoclopramide hydrochloride tablet
- NDC Code(s): 62559-165-01, 62559-166-01
- Packager: ANI Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 19, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use REGLAN TABLETS safely and effectively. See full prescribing information for REGLAN TABLETS.
REGLAN® (metoclopramide) tablets, for oral use
Initial U.S. Approval: 1979WARNING: TARDIVE DYSKINESIA
See full prescribing information for complete boxed warning.
- •
- Reglan can cause tardive dyskinesia (TD), a serious movement disorder that is often irreversible. There is no known treatment for TD. The risk of developing TD increases with duration of treatment and total cumulative dosage (5.1)
- •
- Discontinue Reglan in patients who develop signs or symptoms of TD (5.1)
- •
- Avoid treatment with Reglan for longer than 12 weeks because of the risk of developing TD with longer-term use (5.1, 2.1, 2.2, 2.3)
RECENT MAJOR CHANGES
Boxed Warning 8/2017
Indications and Usage (1) 8/2017
Dosage and Administration, Dosage for Gastroesophageal Reflux (2.2) 8/2017
Dosage and Administration, Dosage for Acute and Recurrent Diabetic Gastroparesis (2.3) 8/2017
Contraindications (4) 8/2017
Warnings and Precautions, Tardive Dyskinesia (5.1) 8/2017
Warnings and Precautions, Other Extrapyramidal Symptoms (5.2) 8/2017
Warnings and Precautions, Neuroleptic Malignant Syndrome (5.3) 8/2017
Warnings and Precautions, Hyperprolactinemia (5.7) 8/2017
INDICATIONS AND USAGE
Reglan tablets are indicated for the:
- •
- Treatment for 4 to 12 weeks of symptomatic, documented gastroesophageal reflux in adults who fail to respond to conventional therapy (1)
- •
- Relief of symptoms in adults with acute and recurrent diabetic gastroparesis (1)
Limitations of Use:
Reglan tablets are not recommended for use in pediatric patients due to the risk of tardive dyskinesia (TD) and other extrapyramidal symptoms as well as the risk of methemoglobinemia in neonates (1, 8.4)
DOSAGE AND ADMINISTRATION
Gastroesophageal Reflux (2.2)
- •
- Administer Reglan continuously or intermittently:
○ Continuous: Administer 10 to 15 mg, 30 minutes before each meal and at bedtime (maximum of 60 mg per day) for 4 to 12 weeks.
○ Intermittent: Single doses up to 20 mg prior to provoking situation.
Acute and Recurrent Diabetic Gastroparesis (2.3)
- •
- Administer 10 mg, 30 minutes before each meal and at bedtime (maximum of 40 mg per day) for 2 to 8 weeks
Dosage Adjustment in Specific Populations (2.2, 2.3)
- •
- For gastroesophageal reflux and acute and recurrent diabetic gastroparesis, see Full Prescribing Information for recommended dosage reductions for elderly patients, in patients with moderate or severe hepatic or renal impairment, and cytochrome P450 2D6 (CYP2D6) poor metabolizers.
DOSAGE FORMS AND STRENGTHS
Tablets: 5 mg and 10 mg metoclopramide (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- •
- Tardive Dyskinesia (TD), Other Extrapyramidal Symptoms (EPS), and Neuroleptic Malignant Syndrome (NMS): Avoid concomitant use of other drugs known to cause TD/EPS/NMS and avoid use in patients with Parkinson’s Disease. If symptoms occur, discontinue Reglan and seek immediate medical attention (5.1, 5.2, 5.3, 7.1, 7.2)
- •
- Depression and suicidal ideation/suicide: Avoid use. (5.4)
ADVERSE REACTIONS
- •
- Most common adverse reactions (> 10%) are restlessness, drowsiness, fatigue, and lassitude. (6)
To report SUSPECTED ADVERSE REACTIONS, contact ANI Pharmaceuticals, Inc. at 1-800-308-6755 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- •
- Antipsychotics: Potential for additive effects, including TD, EPS, and NMS; avoid concomitant use. (7.1)
- •
- CNS depressants: Increased risk of CNS depression. Avoid concomitant use and monitor for adverse reactions. (7.1)
- •
- Strong CYP2D6 inhibitors (e.g., quinidine, bupropion, fluoxetine, and paroxetine): See Full Prescribing Information for recommended dosage reductions. (2.2, 2.3, 7.1)
- •
- MAO inhibitors: Increased risk of hypertension; avoid concomitant use. (5.5, 7.1)
- •
- Additional drug interactions: See Full Prescribing Information. (7.1, 7.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 8/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: TARDIVE DYSKINESIA
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Dosage for Gastroesophageal Reflux
2.3 Dosage for Acute and Recurrent Diabetic Gastroparesis
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Tardive Dyskinesia
5.2 Other Extrapyramidal Symptoms
5.3 Neuroleptic Malignant Syndrome
5.4 Depression
5.5 Hypertension
5.6 Fluid Retention
5.7 Hyperprolactinemia
5.8 Effects on the Ability to Drive and Operate Machinery
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on Metoclopramide
7.2 Effects of Metoclopramide on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
8.8 NADH-Cytochrome b5 Reductase Deficiency
8.9 CYP2D6 Poor Metabolizers
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: TARDIVE DYSKINESIA
- •
- Reglan can cause tardive dyskinesia (TD), a serious movement disorder that is often irreversible. There is no known treatment for TD. The risk of developing TD increases with duration of treatment and total cumulative dosage [see Warnings and Precautions (5.1)].
- •
- Discontinue Reglan in patients who develop signs or symptoms of TD. In some patients, symptoms may lessen or resolve after Reglan is stopped [see Warnings and Precautions (5.1)].
- •
- Avoid treatment with Reglan for longer than 12 weeks because of the increased risk of developing TD with longer-term use [see Warnings and Precautions (5.1) and Dosage and Administration (2.2, 2.3)].
-
1 INDICATIONS AND USAGE
Reglan tablets are indicated for the:
- •
- Treatment for 4 to 12 weeks of symptomatic, documented gastroesophageal reflux in adults who fail to respond to conventional therapy.
- •
- Relief of symptoms in adults with acute and recurrent diabetic gastroparesis.
Limitations of Use:
Reglan tablets are not recommended for use in pediatric patients due to the risk of developing tardive dyskinesia (TD) and other extrapyramidal symptoms as well as the risk of methemoglobinemia in neonates [see Use in Specific Populations (8.4)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
Avoid treatment with Reglan for longer than 12 weeks because of the increased risk of developing TD with longer-term use [see Dosage and Administration (2.2, 2.3), Warnings and Precautions (5.1)].
2.2 Dosage for Gastroesophageal Reflux
Reglan tablets may be administered continuously or intermittently in patients with symptomatic gastroesophageal reflux who fail to respond to conventional therapy:
Continuous Dosing
The recommended adult dosage of Reglan is 10 to 15 mg four times daily for 4 to 12 weeks. The treatment duration is determined by endoscopic response. Administer the dosage thirty minutes before each meal and at bedtime. The maximum recommended daily dosage is 60 mg.
Table 1 displays the recommended daily dosage and maximum daily dosage for adults and dosage adjustments for patients with moderate or severe hepatic impairment (Child-Pugh B or C), in patients with creatinine clearance less than 60 mL/minute, in cytochrome P450 2D6 (CYP2D6) poor metabolizers, and with concomitant use with strong CYP2D6 inhibitors.
Intermittent Dosing
If symptoms only occur intermittently or at specific times of the day, administer Reglan in single dose up to 20 mg prior to the provoking situation. Consider dosage reductions for the populations and situations in Table 1.
- Table 1. Recommended Reglan Tablet Dosage in Patients with Gastroesophageal Reflux
Recommended Dosage
Maximum Recommended Daily Dosage
Adult patients
10 to 15 mg four times daily (thirty minutes before each meal and at bedtime)
60 mg
Mild hepatic impairment (Child-Pugh A)
Elderly patients [see Use in Specific Populations (8.5)]
5 mg1 four times daily (thirty minutes before each meal and at bedtime)
Moderate or severe hepatic impairment (Child-Pugh B or C) [see Use in Specific Populations (8.7)]
5 mg four times daily (thirty minutes before each meal and at bedtime), or
10 mg taken three times daily
30 mg
CYP2D6 poor metabolizers [see Use in Specific Populations (8.9)]
Concomitant use with strong CYP2D6 inhibitors (e.g., quinidine, bupropion, fluoxetine, and paroxetine) [see Drug Interactions (7.1)]
Moderate or severe renal impairment (creatinine clearance less than or equal to 60 mL/minute) [see Use in Specific Populations (8.6)]
Patients with End-Stage Renal Disease (ESRD) including those treated with hemodialysis and continuous ambulatory peritoneal dialysis [see Use in Specific Populations (8.6)]
5 mg four times daily (thirty minutes before each meal and at bedtime) or 10 mg twice daily
20 mg
1 Elderly patients may be more sensitive to the therapeutic or adverse effects of Reglan; therefore, consider a lower starting dosage of 5 mg four times daily with titration to the recommended adult dosage of 10 to 15 mg four times daily based upon response and tolerability.
2.3 Dosage for Acute and Recurrent Diabetic Gastroparesis
The recommended adult dosage for the treatment of acute and recurrent diabetic gastroparesis is 10 mg four times daily for 2 to 8 weeks, depending on symptomatic response. Avoid Reglan treatment for greater than 12 weeks [see Warnings and Precautions (5.1)]. Administer the dosage thirty minutes before each meal and at bedtime. The maximum recommended daily dosage is 40 mg.
Table 2 displays the recommended daily dosage and maximum daily dosage for adults and dosage adjustments for patients with moderate or severe hepatic impairment (Child-Pugh B or C), in patients with creatinine clearance less than 60 mL/minute, in cytochrome P450 2D6 (CYP2D6) poor metabolizers, and with concomitant use with strong CYP2D6 inhibitors.
If patients with diabetic gastroparesis have severe nausea or vomiting and are unable to take oral Reglan tablets, consider starting therapy with metoclopramide injection given intramuscularly or intravenously for up to 10 days (see the prescribing information for metoclopramide injection). After patients are able to take oral therapy, switch to Reglan tablets.
- Table 2. Recommended Reglan Tablet Dosage in Patients with Acute and Recurrent Diabetic Gastroparesis
Recommended Dosage
Maximum Recommended Daily Dosage
Adult Patients
10 mg four times daily (30 minutes before each meal and at bedtime)
40 mg
Mild hepatic impairment (Child-Pugh A)
Elderly patients [see Use in Specific Populations (8.5)]
5 mg1 four times daily (30 minutes before each meal and at bedtime)
Moderate or severe hepatic impairment (Child-Pugh B or C) [see Use in Specific Populations (8.7)]
5 mg four times daily (30 minutes before each meal and at bedtime)
20 mg
CYP2D6 poor metabolizers [see Use in Specific Populations (8.9)]
Concomitant use with strong CYP2D6 inhibitors (e.g., quinidine). Avoid use with bupropion, fluoxetine, and paroxetine [see Drug Interactions (7.1)]
Moderate or severe renal impairment (creatinine clearance less than 60 mL/minute) [see Use in Specific Populations (8.6)]
Patients with End-Stage Renal Disease (ESRD) including those treated with hemodialysis and continuous ambulatory peritoneal dialysis [see Use in Specific Populations (8.6)]
5 mg twice daily
10 mg
- 1 Elderly patients may be more sensitive to the therapeutic or adverse effects of Reglan; therefore, consider a lower dosage of 5 mg four times daily with titration to the recommended adult dosage of 10 mg four times daily based upon response and tolerability.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Reglan is contraindicated:
- •
- In patients with a history of tardive dyskinesia (TD) or a dystonic reaction to metoclopramide [see Warnings and Precautions (5.1, 5.2)].
- •
- When stimulation of gastrointestinal motility might be dangerous (e.g., in the presence of gastrointestinal hemorrhage, mechanical obstruction, or perforation).
- •
- In patients with pheochromocytoma or other catecholamine-releasing paragangliomas. Reglan may cause a hypertensive/pheochromocytoma crisis, probably due to release of catecholamines from the tumor [see Warnings and Precautions (5.5)].
- •
- In patients with epilepsy. Reglan may increase the frequency and severity of seizures [see Adverse Reactions (6)].
- •
- In patients with hypersensitivity to metoclopramide. Reactions have included laryngeal and glossal angioedema and bronchospasm [see Adverse Reactions (6)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Tardive Dyskinesia
Metoclopramide can cause tardive dyskinesia (TD), a syndrome of potentially irreversible and disfiguring involuntary movements of the face or tongue, and sometimes of the trunk and/or extremities. Movements may be choreoathetotic in appearance. The risk of developing TD and the likelihood that TD will become irreversible increases with duration of treatment and total cumulative dosage. Additionally, the risk of developing TD is increased among the elderly, especially elderly women [see Use in Specific Populations (8.5)], and in patients with diabetes mellitus. Due to the risk of developing TD, avoid treatment with Reglan for longer than 12 weeks and reduce the dosage in elderly patients [see Dosage and Administration (2.2, 2.3)].
Discontinue Reglan immediately in patients who develop signs and symptoms of TD. There is no known effective treatment for established cases of TD, although in some patients TD may remit, partially or completely, within several weeks to months after Reglan is withdrawn.
Reglan itself may suppress, or partially suppress, the signs of TD, thereby masking the underlying disease process. The effect of this symptomatic suppression upon the long-term course of TD is unknown. Reglan is contraindicated in patients with a history of TD [see Contraindications (4)]. Avoid Reglan in patients receiving other drugs that are likely to cause TD (e.g., antipsychotics).
5.2 Other Extrapyramidal Symptoms
In addition to TD, metoclopramide may cause other extrapyramidal symptoms (EPS), parkinsonian symptoms, and motor restlessness. Advise patients to seek immediate medical attention if such symptoms occur and to discontinue Reglan.
- •
- Extrapyramidal symptoms (EPS), such as acute dystonic reactions, occurred in patients treated with metoclopramide dosages of 30 mg to 40 mg daily. Such reactions occurred more frequently in adults less than 30 years of age and at higher than recommended dosages. EPS occurred more frequently in pediatric patients compared to adults (Reglan is not approved for use in pediatric patients). Symptoms can occur in the first 24 to 48 hours after starting metoclopramide. Symptoms included involuntary movements of limbs and facial grimacing, torticollis, oculogyric crisis, rhythmic protrusion of tongue, bulbar type of speech, trismus, or dystonic reactions resembling tetanus. Rarely, dystonic reactions were present as stridor and dyspnea, possibly due to laryngospasm. Diphenhydramine hydrochloride or benztropine mesylate may be used to treat these adverse reactions. Avoid Reglan in patients receiving other drugs that can cause EPS (e.g., antipsychotics).
- •
- Parkinsonian symptoms (bradykinesia, tremor, cogwheel rigidity, mask-like facies) have occurred after starting metoclopramide, more commonly within the first 6 months, but also after longer periods. Symptoms generally have subsided within 2 to 3 months after discontinuation of Reglan. Avoid Reglan in patients with Parkinson’s disease and other patients being treated with antiparkinsonian drugs due to potential exacerbation of symptoms. Avoid treatment with Reglan for more than 12 weeks [see Dosage and Administration (2.2, 2.3), Warnings and Precautions (5.1)].
- •
- Motor restlessness (akathisia) has developed and consisted of feelings of anxiety, agitation, jitteriness, and insomnia, as well as inability to sit still, pacing, and foot tapping. If symptoms resolve, consider restarting at a lower dosage.
5.3 Neuroleptic Malignant Syndrome
Metoclopramide may cause a potentially fatal symptom complex called neuroleptic malignant syndrome (NMS). NMS has been reported in association with metoclopramide overdosage and concomitant treatment with another drug associated with NMS. Avoid Reglan in patients receiving other drugs associated with NMS, including typical and atypical antipsychotics.
Clinical manifestations of NMS include hyperpyrexia, muscle rigidity, altered mental status, and manifestations of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac arrhythmias). Additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure. Patients with such symptoms should be evaluated immediately.
In the diagnostic evaluation, consider the presence of other serious medical conditions (e.g., pneumonia, systemic infection) and untreated or inadequately treated extrapyramidal signs and symptoms. Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, malignant hyperthermia, drug fever, serotonin syndrome, and primary central nervous system pathology.
Management of NMS includes:
- •
- Immediate discontinuation of Reglan and other drugs not essential to concurrent therapy [see Drug Interactions (7.1)].
- •
- Intensive symptomatic treatment and medical monitoring.
- •
- Treatment of any concomitant serious medical problems for which specific treatments are available.
5.4 Depression
Depression has occurred in metoclopramide-treated patients with and without a history of depression. Symptoms have included suicidal ideation and suicide. Avoid Reglan use in patients with a history of depression.
5.5 Hypertension
Metoclopramide may elevate blood pressure. In one study in hypertensive patients, intravenously administered metoclopramide was shown to release catecholamines; hence, avoid use in patients with hypertension or in patients taking monoamine oxidase inhibitors [see Drug Interactions (7.1)].
There are also clinical reports of hypertensive crises in patients with undiagnosed pheochromocytoma. Reglan is contraindicated in patients with pheochromocytoma or other catecholamine-releasing paragangliomas [see Contraindications (4)]. Discontinue Reglan in any patient with a rapid rise in blood pressure.
5.6 Fluid Retention
Because Reglan produces a transient increase in plasma aldosterone, patients with cirrhosis or congestive heart failure may be at risk of developing fluid retention and volume overload. Discontinue Reglan if any of these adverse reactions occur.
5.7 Hyperprolactinemia
As with other dopamine D2 receptor antagonists, metoclopramide elevates prolactin levels.
Hyperprolactinemia may suppress hypothalamic GnRH, resulting in reduced pituitary gonadotropin secretion. This, in turn, may inhibit reproductive function by impairing gonadal steroidogenesis in both female and male patients. Galactorrhea, amenorrhea, gynecomastia, and impotence have been reported with prolactin-elevating drugs, including metoclopramide.
Hyperprolactinemia may potentially stimulate prolactin-dependent breast cancer. However, some clinical studies and epidemiology studies have not shown an association between administration of dopamine D2 receptor antagonists and tumorigenesis in humans [see Nonclinical Toxicology (13.1)].
5.8 Effects on the Ability to Drive and Operate Machinery
Metoclopramide may impair the mental and/or physical abilities required for the performance of hazardous tasks such as operating machinery or driving a motor vehicle. Concomitant use of central nervous system (CNS) depressants or drugs associated with EPS may increase this effect (e.g., alcohol, sedatives, hypnotics, opiates, and anxiolytics). Avoid Reglan or the interacting drug, depending on the importance of the drug to the patient [see Drug Interactions (7.1)].
-
6 ADVERSE REACTIONS
The following adverse reactions are described, or described in greater detail, in other sections of the labeling:
- •
- Tardive dyskinesia [see Boxed Warning and Warnings and Precautions (5.1)]
- •
- Other extrapyramidal effects [see Warnings and Precautions (5.2)]
- •
- Neuroleptic malignant syndrome [see Warnings and Precautions (5.3)]
- •
- Depression [see Warnings and Precautions (5.4)]
- •
- Hypertension [see Warnings and Precautions (5.5)]
- •
- Fluid retention [see Warnings and Precautions (5.6)]
- •
- Hyperprolactinemia [see Warnings and Precautions (5.7)]
- •
- Effects on the ability to drive and operate machinery [see Warnings and Precautions (5.8)]
The following adverse reactions have been identified from clinical studies or postmarketing reports of metoclopramide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The most common adverse reactions (in approximately 10% of patients receiving 10 mg of metoclopramide four times daily) were restlessness, drowsiness, fatigue, and lassitude. In general, the incidence of adverse reactions correlated with the dosage and duration of metoclopramide administration.
Adverse reactions, especially those involving the nervous system, occurred after stopping metoclopramide including dizziness, nervousness, and headaches.
Central Nervous System Disorders
- •
- Tardive dyskinesia, acute dystonic reactions, drug-induced parkinsonism, akathisia, and other extrapyramidal symptoms
- •
- Convulsive seizures
- •
- Hallucinations
- •
- Restlessness, drowsiness, fatigue, and lassitude occurred in approximately 10% of patients who received 10 mg four times daily. Insomnia, headache, confusion, dizziness, or depression with suicidal ideation occurred less frequently.
- •
- Neuroleptic malignant syndrome, serotonin syndrome (in combination with serotonergic agents).
Endocrine Disorders: Fluid retention secondary to transient elevation of aldosterone. Galactorrhea, amenorrhea, gynecomastia, impotence secondary to hyperprolactinemia
Cardiovascular Disorders: Acute congestive heart failure, possible atrioventricular block, hypotension, hypertension, supraventricular tachycardia, bradycardia, fluid retention
Gastrointestinal Disorders: Nausea, bowel disturbances (primarily diarrhea)
Hepatic Disorders: Hepatotoxicity, characterized by, e.g., jaundice and altered liver function tests, when metoclopramide was administered with other drugs with known hepatotoxic potential
Renal and Urinary Disorders: Urinary frequency, urinary incontinence
Hematologic Disorders: Agranulocytosis, neutropenia, leukopenia, methemoglobinemia, sulfhemoglobinemia
Hypersensitivity Reactions: Bronchospasm (especially in patients with a history of asthma), urticaria; rash; angioedema, including glossal or laryngeal edema
Eye Disorders: Visual disturbances
Metabolism Disorders: Porphyria
-
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on Metoclopramide
Table 3 displays the effects of other drugs on metoclopramide.
Table 3. Effects of Other Drugs on Metoclopramide
Antipsychotics
Clinical Impact
Potential for additive effects, including increased frequency and severity of tardive dyskinesia (TD), other extrapyramidal symptoms (EPS), and neuroleptic malignant syndrome (NMS).
Intervention
Avoid concomitant use [see Warnings and Precautions (5.1, 5.2, 5.3)].
Strong CYP2D6 Inhibitors, not Included in Antipsychotic Category Above
Clinical Impact
Increased plasma concentrations of metoclopramide; risk of exacerbation of extrapyramidal symptoms [see Clinical Pharmacology (12.3)].
Intervention
Reduce the Reglan dosage [see Dosage and Administration (2.2, 2.3)].
Examples
quinidine, bupropion, fluoxetine, and paroxetine
Monoamine Oxidase Inhibitors
Clinical Impact
Increased risk of hypertension [see Warnings and Precautions (5.5)].
Intervention
Avoid concomitant use.
Central Nervous System (CNS) Depressants
Clinical Impact
Increased risk of CNS depression [see Warnings and Precautions (5.8)].
Intervention
Avoid Reglan or the interacting drug, depending on the importance of the drug to the patient.
Examples
alcohol, sedatives, hypnotics, opiates and anxiolytics
Drugs that Impair Gastrointestinal Motility
Clinical Impact
Decreased systemic absorption of metoclopramide.
Intervention
Monitor for reduced therapeutic effect.
Examples
antiperistaltic antidiarrheal drugs, anticholinergic drugs, and opiates
Dopaminergic Agonists and Other Drugs that Increase Dopamine Concentrations
Clinical Impact
Decreased therapeutic effect of metoclopramide due to opposing effects on dopamine.
Intervention
Monitor for reduced therapeutic effect.
Examples
apomorphine, bromocriptine, cabergoline, levodopa, pramipexole, ropinirole, and rotigotine
7.2 Effects of Metoclopramide on Other Drugs
Table 4 displays the effects of Metoclopramide on other drugs.
Table 4. Effects of Metoclopramide on Other Drugs
Dopaminergic Agonists and Drugs Increasing Dopamine Concentrations
Clinical Impact
Opposing effects of metoclopramide and the interacting drug on dopamine. Potential exacerbation of symptoms (e.g., parkinsonian symptoms).
Intervention
Avoid concomitant use [see Warnings and Precautions (5.2)].
Examples
Apomorphine, bromocriptine, cabergoline, levodopa, pramipexole, ropinirole, rotigotine
Succinylcholine, Mivacurium
Clinical Impact
Metoclopramide inhibits plasma cholinesterase leading to enhanced neuromuscular blockade.
Intervention
Monitor for signs and symptoms of prolonged neuromuscular blockade.
Drugs with Absorption Altered due to Increased Gastrointestinal Motility
Clinical Impact
The effect of metoclopramide on other drugs is variable. Increased gastrointestinal (GI) motility by metoclopramide may impact absorption of other drugs leading to decreased or increased drug exposure.
Intervention
Drugs with Decreased Absorption (e.g., digoxin, atovaquone, posaconazole oral suspension*, fosfomycin): Monitor for reduced therapeutic effect of the interacting drug. For digoxin monitor therapeutic drug concentrations and increase the digoxin dose as needed (see prescribing information for digoxin).
Drugs with Increased Absorption (e.g., sirolimus, tacrolimus, cyclosporine): Monitor therapeutic drug concentrations and adjust the dose as needed. See prescribing information for the interacting drug.
Insulin
Clinical Impact
Increased GI motility by metoclopramide may increase delivery of food to the intestines and increase blood glucose.
Intervention
Monitor blood glucose and adjust insulin dosage regimen as needed.
* Interaction does not apply to posaconazole delayed-release tablets
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Published studies, including retrospective cohort studies, national registry studies, and meta-analyses, do not report an increased risk of adverse pregnancy-related outcomes with use of metoclopramide during pregnancy.
There are potential risks to the neonate following exposure in utero to metoclopramide during delivery [see Clinical Considerations]. In animal reproduction studies, no adverse developmental effects were observed with oral administration of metoclopramide to pregnant rats and rabbits at exposures about 6 and 12 times the maximum recommended human dose (MRHD) [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defects, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in the clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Metoclopramide crosses the placental barrier and may cause extrapyramidal signs and methemoglobinemia in neonates with maternal administration during delivery. Monitor neonates for extrapyramidal signs [see Warnings and Precautions (5.1, 5.2), Use in Specific Populations (8.4)].
Data
Animal Data
Reproduction studies have been performed following administration of oral metoclopramide during organogenesis in pregnant rats at about 6 times the MRHD calculated on body surface area and in pregnant rabbits at about 12 times the MRHD calculated on body surface area. No evidence of adverse developmental effects due to metoclopramide were observed.
8.2 Lactation
Risk Summary
Limited published data report the presence of metoclopramide in human milk in variable amounts. Breastfed infants exposed to metoclopramide have experienced gastrointestinal adverse reactions, including intestinal discomfort and increased intestinal gas formation [see Data]. Metoclopramide elevates prolactin levels [see Warnings and Precautions (5.7)]; however, the published data are not adequate to support drug effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Reglan and any potential adverse effects on the breastfed child from Reglan or from the underlying maternal condition.
Clinical Considerations
Monitor breastfeeding neonates because metoclopramide may cause extrapyramidal signs (dystonias) and methemoglobinemia [see Warnings and Precautions (5.1, 5.2), Use in Specific Populations (8.4)].
Data
In published clinical studies, the estimated amount of metoclopramide received by the breastfed infant was less than 10% of the maternal weight-adjusted dose. In one study, the estimated daily amount of metoclopramide received by infants from breast milk ranged from 6 to 24 mcg/kg/day in early puerperium (3 to 9 days postpartum) and from 1 to 13 mcg/kg/day at 8 to 12 weeks postpartum.
8.4 Pediatric Use
Reglan is not recommended for use in pediatric patients due to the risk of tardive dyskinesia (TD) and other extrapyramidal symptoms as well as the risk of methemoglobinemia in neonates. The safety and effectiveness of Reglan in pediatric patients have not been established.
Dystonias and other extrapyramidal symptoms associated with metoclopramide are more common in pediatric patients than in adults [see Warnings and Precautions (5.1, 5.2)]. In addition, neonates have reduced levels of NADH-cytochrome b5 reductase, making them more susceptible to methemoglobinemia, a possible adverse reaction of metoclopramide use in neonates [see Use in Specific Populations (8.8)].
8.5 Geriatric Use
Metoclopramide is known to be substantially excreted by the kidney, and the risk of adverse reactions, including tardive dyskinesia (TD), may be greater in patients with impaired renal function [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)]. Elderly patients are more likely to have decreased renal function and may be more sensitive to the therapeutic or adverse effects of metoclopramide; therefore, consider a reduced dosage of Reglan in elderly patients [see Boxed Warning, Dosage and Administration (2.2, 2.3), Warnings and Precautions (5.1)].
8.6 Renal Impairment
The clearance of metoclopramide is decreased and the systemic exposure is increased in patients with moderate to severe renal impairment compared to patients with normal renal function, which may increase the risk of adverse reactions. Reduce the Reglan dosage in patients with moderate and severe renal impairment (creatinine clearance less than or equal to 60 mL/minute), including those receiving hemodialysis and continuous ambulatory peritoneal dialysis [see Dosage and Administration (2.2, 2.3), Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Patients with severe hepatic impairment (Child-Pugh C) have reduced systemic metoclopramide clearance (by approximately 50%) compared to patients with normal hepatic function. The resulting increase in metoclopramide blood concentrations increases the risk of adverse reactions. There is no pharmacokinetic data in patients with moderate hepatic impairment (Child-Pugh B). Reduce Reglan dosage in patients with moderate or severe (Child-Pugh B or C) hepatic impairment [see Dosage and Administration (2.2, 2.3)]. There is no dosage adjustment required for patients with mild hepatic impairment (Child-Pugh A).
In addition, metoclopramide, by producing a transient increase in plasma aldosterone, may increase the risk of fluid retention in patients with hepatic impairment [see Warnings and Precautions (5.6)].
Monitor patients with hepatic impairment for the occurrence of fluid retention and volume overload.
8.8 NADH-Cytochrome b5 Reductase Deficiency
Metoclopramide-treated patients with NADH-cytochrome b5 reductase deficiency are at an increased risk of developing methemoglobinemia and/or sulfhemoglobinemia. For patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency with metoclopramide-induced methemoglobinemia, methylene blue treatment is not recommended. Methylene blue may cause hemolytic anemia in patients with G6PD deficiency, which may be fatal [see Overdosage (10)].
8.9 CYP2D6 Poor Metabolizers
Metoclopramide is a substrate of CYP2D6. The elimination of metoclopramide may be slowed in patients who are CYP2D6 poor metabolizers (compared to patients who are CYP2D6 intermediate, extensive, or ultra-rapid metabolizers); possibly increasing the risk of dystonic and other adverse reactions to Reglan [see Clinical Pharmacology (12.3)]. Reduce the Reglan dosage in patients who are poor CYP2D6 metabolizers [see Dosage and Administration (2.2, 2.3)].
-
10 OVERDOSAGE
Manifestations of metoclopramide overdosage included drowsiness, disorientation, extrapyramidal reactions, other adverse reactions associated with metoclopramide use (including, e.g., methemoglobinemia), and sometimes death. Neuroleptic malignant syndrome (NMS) has been reported in association with metoclopramide overdose and concomitant treatment with another drug associated with NMS [see Warnings and Precautions (5.1, 5.2, 5.3)].
There are no specific antidotes for Reglan overdosage. If over-exposure occurs, call your Poison Control Center at 1-800-222-1222 for current information on the management of poisoning or overdosage .
Methemoglobinemia can be reversed by the intravenous administration of methylene blue. However, methylene blue may cause hemolytic anemia in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency, which may be fatal.
Hemodialysis and continuous ambulatory peritoneal dialysis do not remove significant amounts of metoclopramide.
-
11 DESCRIPTION
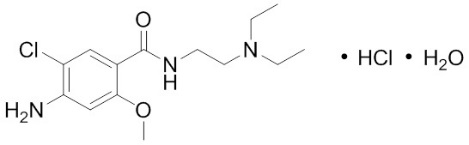
Metoclopramide hydrochloride, the active ingredient of Reglan, is a dopamine-2 receptor antagonist. Metoclopramide hydrochloride (metoclopramide monohydrochloride monohydrate) is a white crystalline, odorless substance, freely soluble in water. Its chemical name is 4-amino-5-chloro-N-[2-(diethylamino)ethyl]-2-methoxy benzamide monohydrochloride monohydrate.
The molecular formula is C14H22ClN3O2•HCl•H2O. Its molecular weight is 354.3. The structural formula is:
Reglan tablets are for oral administration. Reglan is available in 5 mg and 10 mg tablets.
- •
- Each Reglan 5 mg tablet contains 5 mg metoclopramide (equivalent to 5.91 mg of metoclopramide hydrochloride USP). Inactive ingredients consist of corn starch, D&C Yellow 10 Aluminum Lake, FD&C Blue 1 Aluminum Lake, lactose, microcrystalline cellulose, silicon dioxide, and stearic acid.
- •
- Each Reglan 10 mg tablet contains 10 mg metoclopramide (equivalent to 11.82 mg metoclopramide hydrochloride USP). Inactive ingredients consist of magnesium stearate, mannitol, microcrystalline cellulose, and stearic acid.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Metoclopramide stimulates motility of the upper gastrointestinal tract without stimulating gastric, biliary, or pancreatic secretions. The exact mechanism of action of metoclopramide in the treatment of gastroesophageal reflux and acute and recurrent diabetic gastroparesis has not been fully established. It seems to sensitize tissues to the action of acetylcholine. The effect of metoclopramide on motility is not dependent on intact vagal innervation, but it can be abolished by anticholinergic drugs.
Metoclopramide increases the tone and amplitude of gastric (especially antral) contractions, relaxes the pyloric sphincter and the duodenal bulb, and increases peristalsis of the duodenum and jejunum resulting in accelerated gastric emptying and intestinal transit. It increases the resting tone of the lower esophageal sphincter. It has little, if any, effect on the motility of the colon or gallbladder.
12.2 Pharmacodynamics
Gastroesophageal Reflux
In patients with gastroesophageal reflux and low lower esophageal sphincter pressure (LESP), single oral doses of Reglan produced dose-related increases in LESP. Effects began at about 5 mg and increased through 20 mg. The increase in LESP from a 5 mg dose lasted about 45 minutes and that of 20 mg lasted between 2 and 3 hours. Increased rate of stomach emptying was observed with single oral doses of 10 mg.
12.3 Pharmacokinetics
Absorption
Relative to an intravenous dose of 20 mg, the absolute bioavailability of oral metoclopramide is 80% ± 15.5% as demonstrated in a crossover study of 18 subjects. Peak plasma concentrations occurred at about 1 to 2 hours after a single oral dose. Similar time to peak was observed after individual doses at steady state.
In a single dose study of 12 subjects, the area under the drug concentration-time curve increased linearly with doses from 20 to 100 mg (5 times the maximum recommended single dose). Peak concentrations increased linearly with dose; time to peak concentrations remained the same; whole body clearance was unchanged; and the elimination rate remained the same. The mean elimination half-life in subjects with normal renal function was 5 to 6 hours. Linear kinetic processes adequately describe the absorption and elimination of metoclopramide.
Distribution
Metoclopramide is not extensively bound to plasma proteins (about 30%). The whole body volume of distribution is high (about 3.5 L/kg), which suggests extensive distribution of drug to the tissues.
Elimination
- Metabolism: Metoclopramide undergoes enzymatic metabolism via oxidation as well as glucuronide and sulfate conjugation reactions in the liver. Monodeethylmetoclopramide, a major oxidative metabolite, is formed primarily by CYP2D6, an enzyme subject to genetic variability [see Dosage and Administration (2.2, 2.3), Use in Specific Populations (8.9)].
- Excretion: Approximately 85% of the radioactivity of an orally administered dose appeared in the urine within 72 hours. After oral administration of 10 or 20 mg, a mean of 18% and 22% of the dose, respectively, was recovered as free metoclopramide in urine within 36 hours.
Specific Populations
- Patients with Renal Impairment: In a study of 24 patients with varying degrees of renal impairment (moderate, severe, and end-stage renal disease (ESRD) requiring dialysis), the systemic exposure (AUC) of metoclopramide in patients with moderate to severe renal impairment was about 2-fold the AUC in subjects with normal renal function. The AUC of metoclopramide in patients with ESRD on dialysis was about 3.5-fold the AUC in subjects with normal renal function [see Dosage and Administration (2.2, 2.3) and Use in Specific Populations (8.6)].
- Patients with Hepatic Impairment: In a group of 8 patients with severe hepatic impairment (Child-Pugh C), the average metoclopramide clearance was reduced by approximately 50% compared to patients with normal hepatic function [see Dosage and Administration (2.2, 2.3) and Use in Specific Populations (8.7)].
Drug Interaction Studies
- Effect of Metoclopramide on CYP2D6 Substrates
- Although in vitro studies suggest that metoclopramide can inhibit CYP2D6, metoclopramide is unlikely to interact with CYP2D6 substrates in vivo at therapeutically relevant concentrations.
- Effect of CYP2D6 Inhibitors on Metoclopramide
- In healthy subjects, 20 mg of metoclopramide and 60 mg of fluoxetine (a strong CYP2D6 inhibitor) were administered, following prior exposure to 60 mg fluoxetine orally for 8 days. The patients who received concomitant metoclopramide and fluoxetine had a 40% and 90% increase in metoclopramide Cmax and AUC0-∞, respectively, compared to patients who received metoclopramide alone (see Table 5) [see Drug Interactions (7.1)].
Table 5. Metoclopramide Pharmacokinetic Parameters in Healthy Subjects with and without Fluoxetine
Parameter
Metoclopramide alone
(mean ± SD)
Metoclopramide with fluoxetine
(mean ± SD)
Cmax (ng/mL)
44 ±15
62.7 ± 9.2
AUC0-∞ (ngˑh/mL)
313 ± 113
591 ± 140
t1/2 (h)
5.5 ± 1.1
8.5 ± 2.2
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
A 77-week study was conducted in rats with oral metoclopramide doses up to 40 mg/kg/day (about six times the maximum recommended human dose on body surface area basis). Metoclopramide elevated prolactin levels and the elevation persisted during chronic administration. An increase in mammary neoplasms was found in rodents after chronic administration of metoclopramide [see Warnings and Precautions (5.7)]. In a rat model for assessing the tumor promotion potential, a 2-week oral treatment with metoclopramide at a dose of 260 mg/kg/day (about 35 times the maximum recommended human dose based on body surface area) enhanced the tumorigenic effect of N-nitrosodiethylamine.
Mutagenesis
Metoclopramide was positive in the in vitro Chinese hamster lung cell/HGPRT forward mutation assay for mutagenic effects and in the in vitro human lymphocyte chromosome aberration assay for clastogenic effects. It was negative in the in vitro Ames mutation assay, the in vitro unscheduled DNA synthesis assay with rat and human hepatocytes, and the in vivo rat micronucleus assay.
Impairment of Fertility
Metoclopramide at intramuscular doses up to 20 mg/kg/day (about three times the maximum recommended human dose based on body surface area) was found to have no effect on fertility and reproductive performance of male and female rats.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
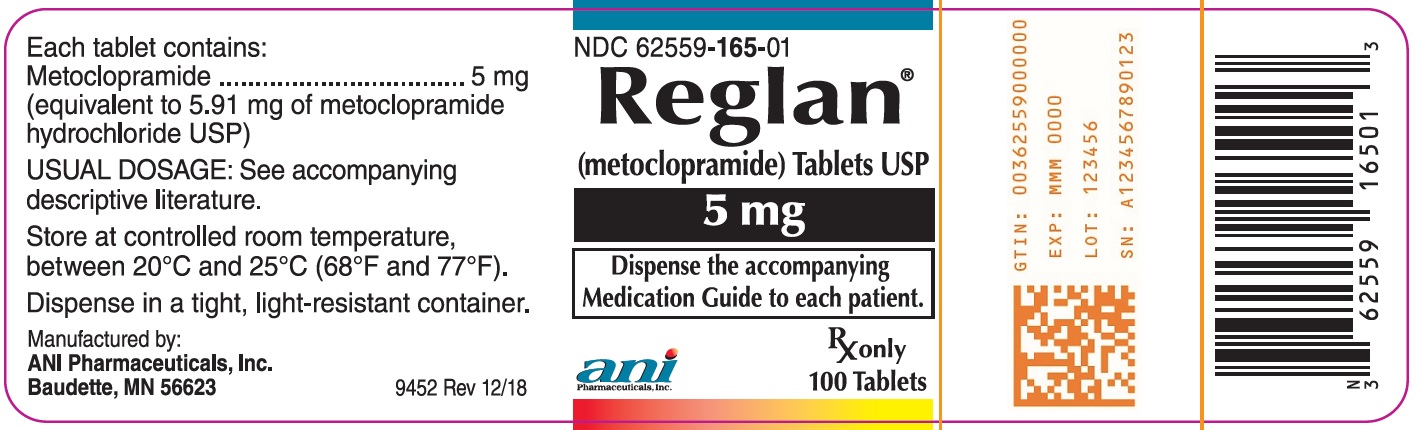
Each green, elliptical-shaped Reglan tablet contains 5 mg metoclopramide. The tablet is debossed “REGLAN” over “5” on one side and “ANI” on the opposite side. Available in bottles of 100 tablets (NDC 62559-165-01)
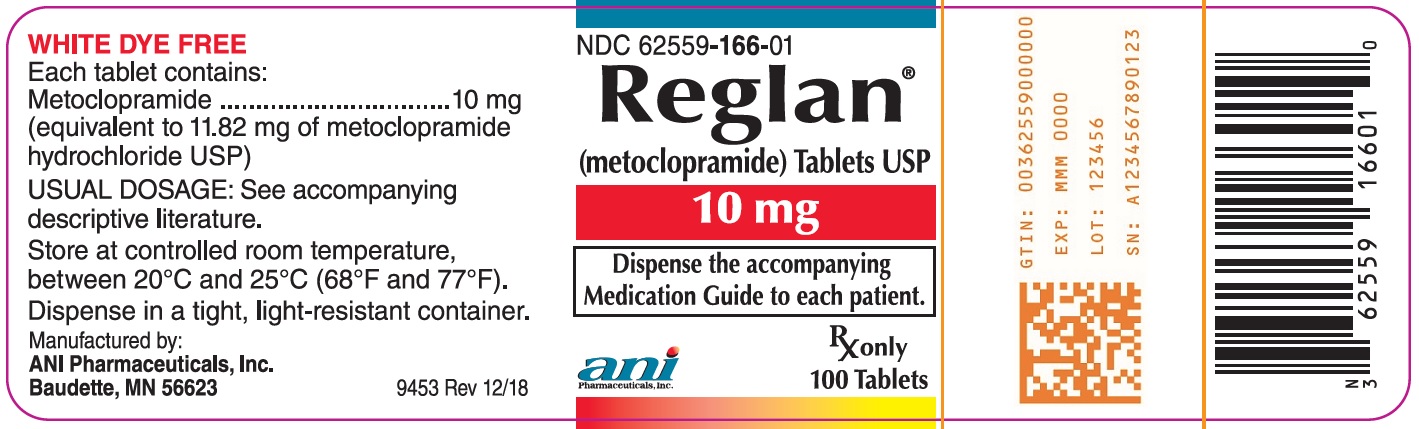
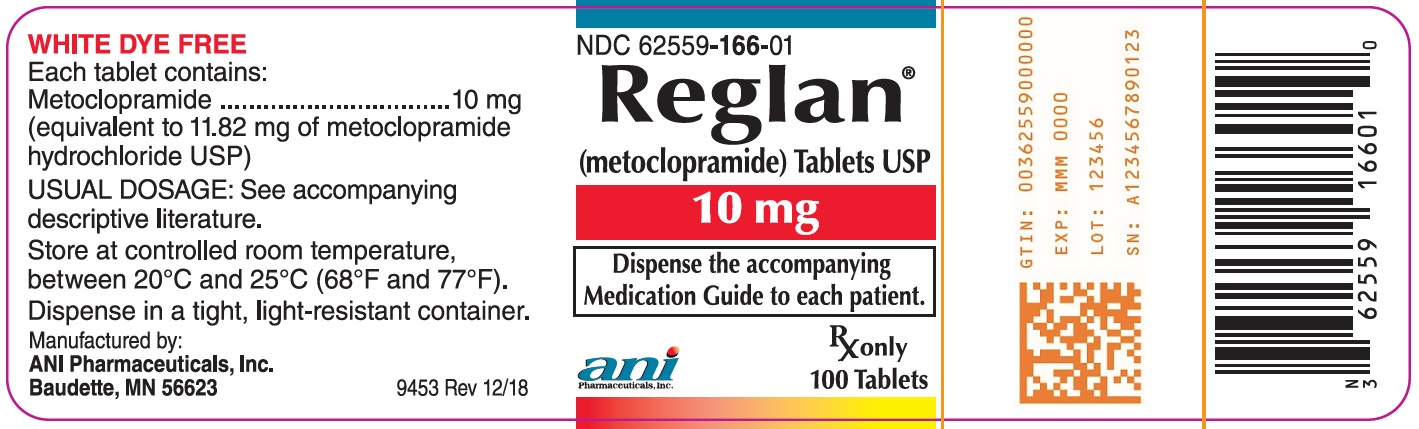
Each white, double edge scored, capsule-shaped Reglan tablet contains 10 mg metoclopramide. The tablet is debossed “REGLAN” on one side and “ANI 10” on the opposite side. Available in bottles of 100 tablets (NDC 62559-166-01)
Dispense tablets in tight, light-resistant container. Store tablets at controlled room temperature between 20°C and 25°C (68°F and 77°F).
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Inform patients or their caregivers that Reglan can cause serious adverse reactions. Instruct patients to discontinue Reglan and contact a healthcare provider immediately if the following serious reactions occur:
- •
- Tardive dyskinesia and other extrapyramidal reactions [see Warnings and Precautions (5.1, 5.2)]
- •
- Neuroleptic malignant syndrome [see Warnings and Precautions (5.3)]
- •
- Depression and/or possible suicidal ideation [see Warnings and Precautions (5.4)]
Inform patients or their caregivers that concomitant treatment with numerous other medications can precipitate or worsen serious adverse reactions such as tardive dyskinesia or other extrapyramidal reactions, neuroleptic malignant syndrome, and CNS depression [see Drug Interactions (7.1, 7.2)]. Explain that the prescriber of any other medication must be made aware that the patient is taking Reglan.
Inform patients or their caregivers that Reglan can cause drowsiness or dizziness, or otherwise impair the mental and/or physical abilities required for the performance of hazardous tasks such as operating machinery or driving a motor vehicle [see Warnings and Precautions (5.8)].
Manufactured by:
ANI Pharmaceuticals, Inc.
Baudette, MN 56623

9454
-
Medication Guide
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised: August 2017 - PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
REGLAN
metoclopramide hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:62559-165 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METOCLOPRAMIDE HYDROCHLORIDE (UNII: W1792A2RVD) (METOCLOPRAMIDE - UNII:L4YEB44I46) METOCLOPRAMIDE 5 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STEARIC ACID (UNII: 4ELV7Z65AP) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) ALUMINUM OXIDE (UNII: LMI26O6933) Product Characteristics Color GREEN Score no score Shape OVAL (elliptical-shaped) Size 10mm Flavor Imprint Code REGLAN5;ANI Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62559-165-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/05/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA017854 07/05/2011 REGLAN
metoclopramide hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:62559-166 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METOCLOPRAMIDE HYDROCHLORIDE (UNII: W1792A2RVD) (METOCLOPRAMIDE - UNII:L4YEB44I46) METOCLOPRAMIDE 10 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color WHITE Score 2 pieces Shape CAPSULE (capsule-shaped) Size 11mm Flavor Imprint Code REGLAN;ANI10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62559-166-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/05/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA017854 07/05/2011 Labeler - ANI Pharmaceuticals, Inc. (145588013)