Label: METRONIDAZOLE cream
- NDC Code(s): 0713-0633-37
- Packager: Cosette Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
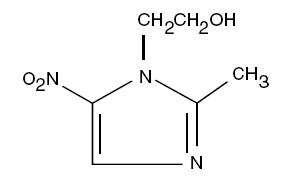
Metronidazole Topical Cream contains metronidazole, USP, at a concentration of 7.5 mg per gram (0.75%) in an emollient cream consisting of emulsifying wax, sorbitol solution, glycerin, isopropyl palmitate, benzyl alcohol, lactic acid and/or sodium hydroxide to adjust pH, and purified water. Metronidazole is a member of the imidazole class of antibacterial agents and is classified therapeutically as an antiprotozoal and antibacterial agent. Chemically, metronidazole is 2-methyl-5-nitro-1 H-imidazole-1-ethanol. The molecular formula is C 6H 9N 3O 3and molecular weight is 171.16. Metronidazole is represented by the following structural formula:
- CLINICAL PHARMACOLOGY
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
PRECAUTIONS
General:
Topical metronidazole has been reported to cause tearing of the eyes. Therefore, contact with the eyes should be avoided. If a reaction suggesting local irritation occurs, patients should be directed to use the medication less frequently or discontinue use. Metronidazole is a nitroimidazole and should be used with care in patients with evidence of, or history of blood dyscrasia.
Information for patients:
This medication is to be used as directed by the physician. It is for external use only. Avoid contact with the eyes.
Drug Interactions:
Oral metronidazole has been reported to potentiate the anticoagulant effect of warfarin and coumarin anticoagulants, resulting in a prolongation of prothrombin time. The effect of topical metronidazole on prothrombin time is not known.
Carcinogenesis, mutagenesis, impairment of fertility :
Metronidazole has shown evidence of carcinogenic activity in a number of studies involving chronic, oral administration in mice and rats but not in studies involving hamsters.
Metronidazole has shown evidence of mutagenic activity in several in vitrobacterial assay systems. In addition, a dose-response increase in the frequency of micronuclei was observed in mice after intraperitoneal injections and an increase in chromosome aberrations have been reported in patients with Crohn's disease who were treated with 200-1200 mg/day of metronidazole for 1 to 24 months. However, no excess chromosomal aberrations in circulating human lymphocytes have been observed in patients treated for 8 months.
Pregnancy:
Teratogenic effects: Pregnancy category B
There are no adequate and well-controlled studies with the use of Metronidazole Topical Cream in pregnant women. Metronidazole crosses the placental barrier and enters the fetal circulation rapidly. No fetotoxicity was observed after oral metronidazole in rats or mice. However, because animal reproduction studies are not always predictive of human response and since oral metronidazole has been shown to be a carcinogen in some rodents, this drug should be used during pregnancy only if clearly needed.
Nursing mothers:
After oral administration, metronidazole is secreted in breast milk in concentrations similar to those found in the plasma. Even though blood levels are significantly lower with topically applied metronidazole than those achieved after oral administration of metronidazole, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
-
ADVERSE REACTIONS
In controlled clinical trials, the total incidence of adverse reactions associated with the use of Metronidazole Topical Cream was approximately10%. Skin discomfort (burning and stinging) was the most frequently reported event followed by erythema, skin irritation, pruritus and worsening of rosacea. All individual events occurred in less than 3% of patients. The following additional adverse experiences have been reported with the topical use of metronidazole: dryness, transient redness, metallic taste, tingling or numbness of extremities and nausea.
To report SUSPECTED ADVERSE REACTIONS, contact Cosette Pharmaceuticals, Inc. at 1-800-922-1038 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- Storage conditions:
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
METRONIDAZOLE
metronidazole creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0713-0633 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METRONIDAZOLE (UNII: 140QMO216E) (METRONIDAZOLE - UNII:140QMO216E) METRONIDAZOLE 7.5 mg in 1 g Inactive Ingredients Ingredient Name Strength SORBITOL (UNII: 506T60A25R) GLYCERIN (UNII: PDC6A3C0OX) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) BENZYL ALCOHOL (UNII: LKG8494WBH) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0713-0633-37 1 in 1 CARTON 03/13/2008 1 45 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077549 03/13/2008 Labeler - Cosette Pharmaceuticals, Inc. (116918230) Registrant - Cosette Pharmaceuticals, Inc. (116918230) Establishment Name Address ID/FEI Business Operations Cosette Pharmaceuticals NC Laboratories, LLC 079419931 analysis(0713-0633) , label(0713-0633) , manufacture(0713-0633) , pack(0713-0633)


