Label: NO MAKEUP BRONZER SPF15- zinc oxide liquid
- NDC Code(s): 45634-711-03
- Packager: N.V Perricone LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DOSAGE & ADMINISTRATION
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- OTHER SAFETY INFORMATION
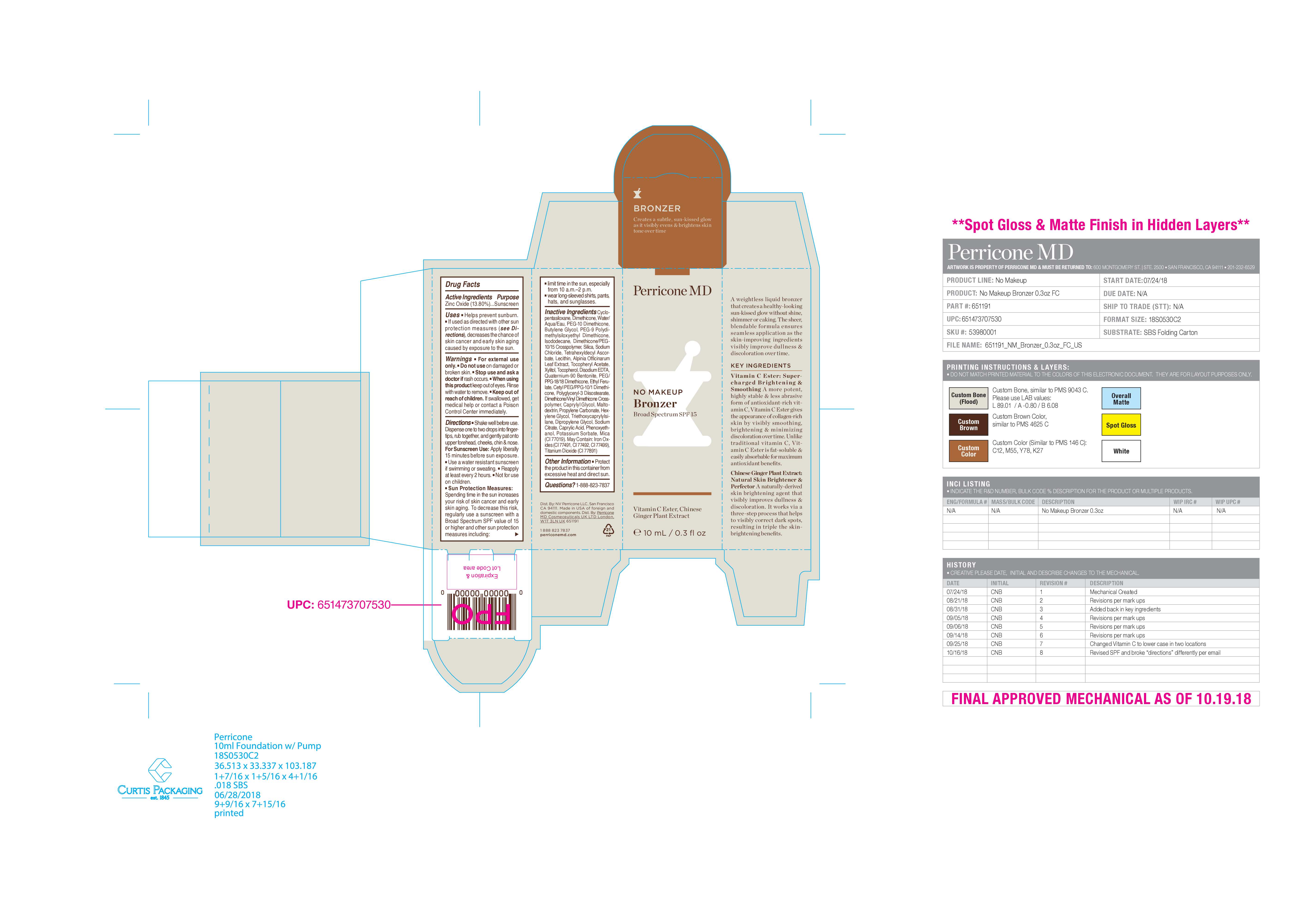
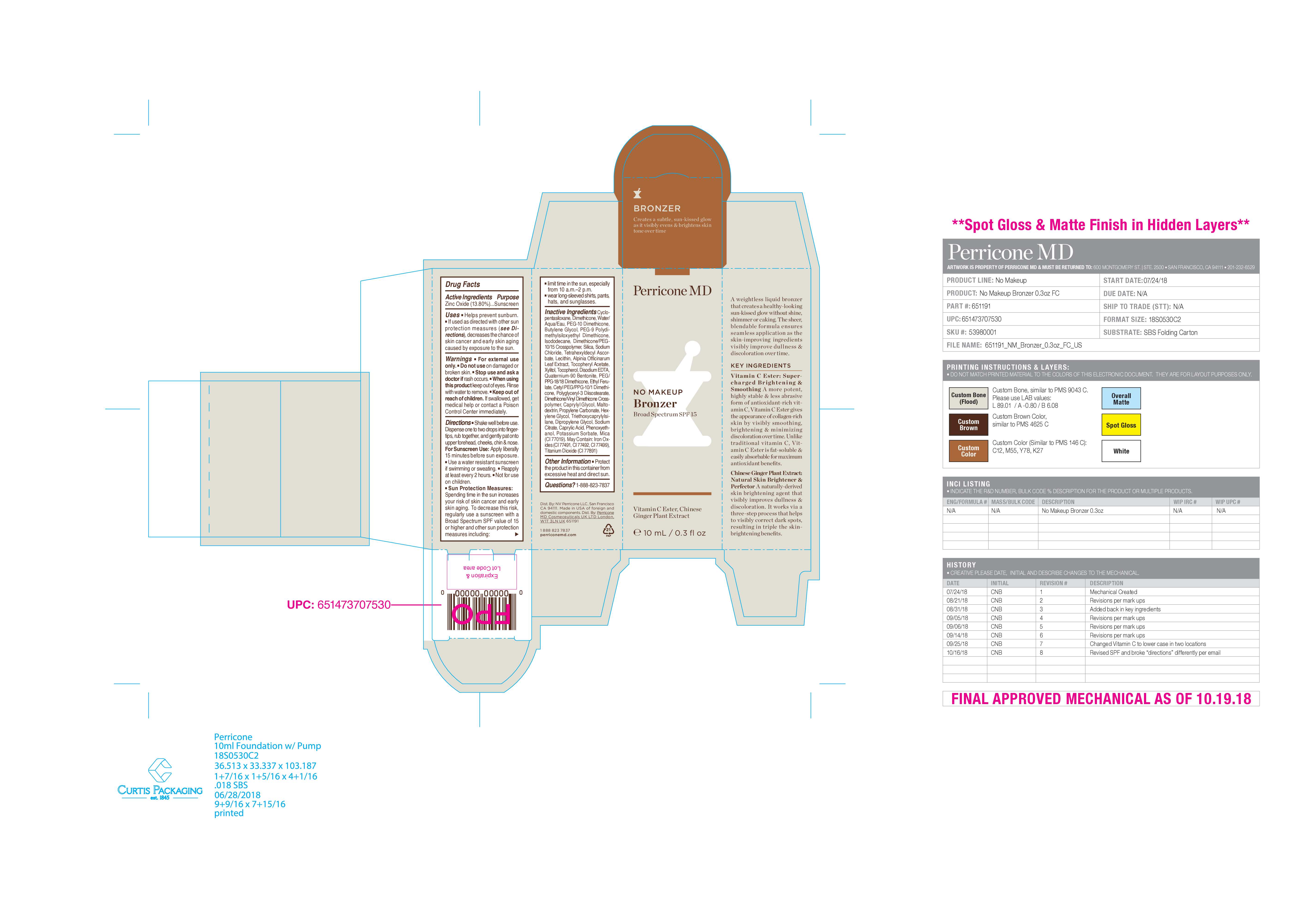
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NO MAKEUP BRONZER SPF15
zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:45634-711 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 1.38 g in 10 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) ISODODECANE (UNII: A8289P68Y2) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ETHYL FERULATE (UNII: 5B8915UELW) PROPYLENE CARBONATE (UNII: 8D08K3S51E) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) FERROUS OXIDE (UNII: G7036X8B5H) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) DIPROPYLENE GLYCOL (UNII: E107L85C40) SODIUM CITRATE (UNII: 1Q73Q2JULR) XYLITOL (UNII: VCQ006KQ1E) CAPRYLIC ACID (UNII: OBL58JN025) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) HEXYLENE GLYCOL (UNII: KEH0A3F75J) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (HARD PARTICLE) (UNII: H895X08VNQ) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) MICA (UNII: V8A1AW0880) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) MALTODEXTRIN (UNII: 7CVR7L4A2D) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) ALPINIA OFFICINARUM LEAF (UNII: 8584IDD6OR) TOCOPHEROL (UNII: R0ZB2556P8) Product Characteristics Color brown Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:45634-711-03 13.9 g in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 05/03/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 05/03/2022 Labeler - N.V Perricone LLC (054414243) Establishment Name Address ID/FEI Business Operations Dimensional Merchandising Inc (DMI) 076693183 manufacture(45634-711)