Label: CETIRIZINE HYDROCHLORIDE (ALLERGY)- cetirizine hydrochloride capsule
- NDC Code(s): 11822-4228-1, 11822-4228-2
- Packager: RITE AID CORPORATION
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 21, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
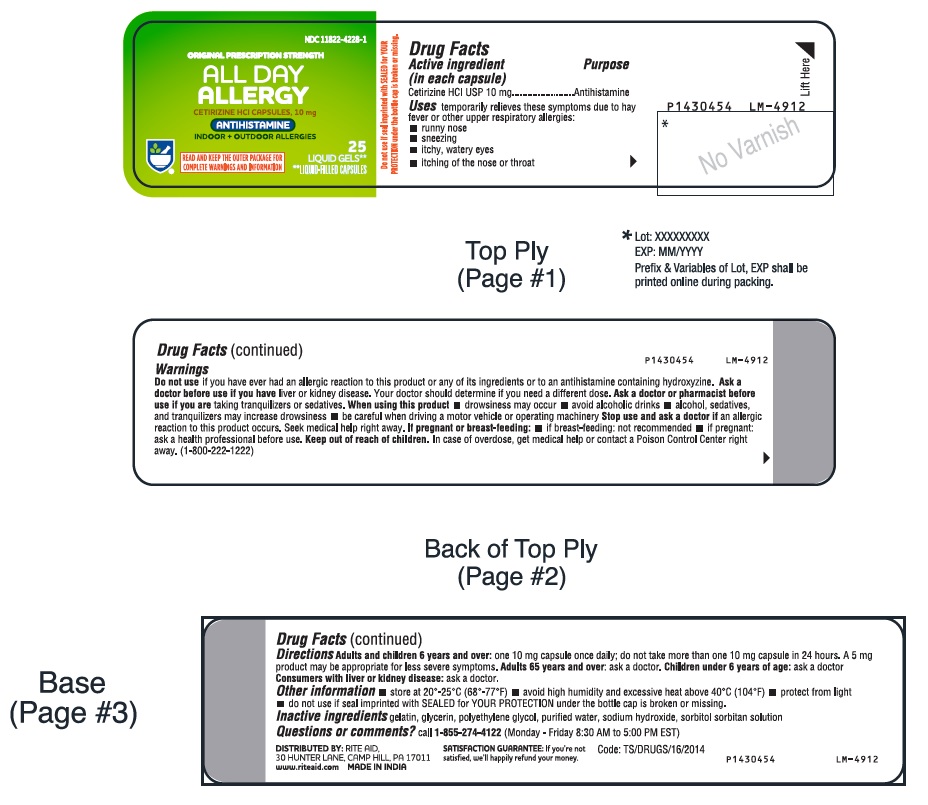
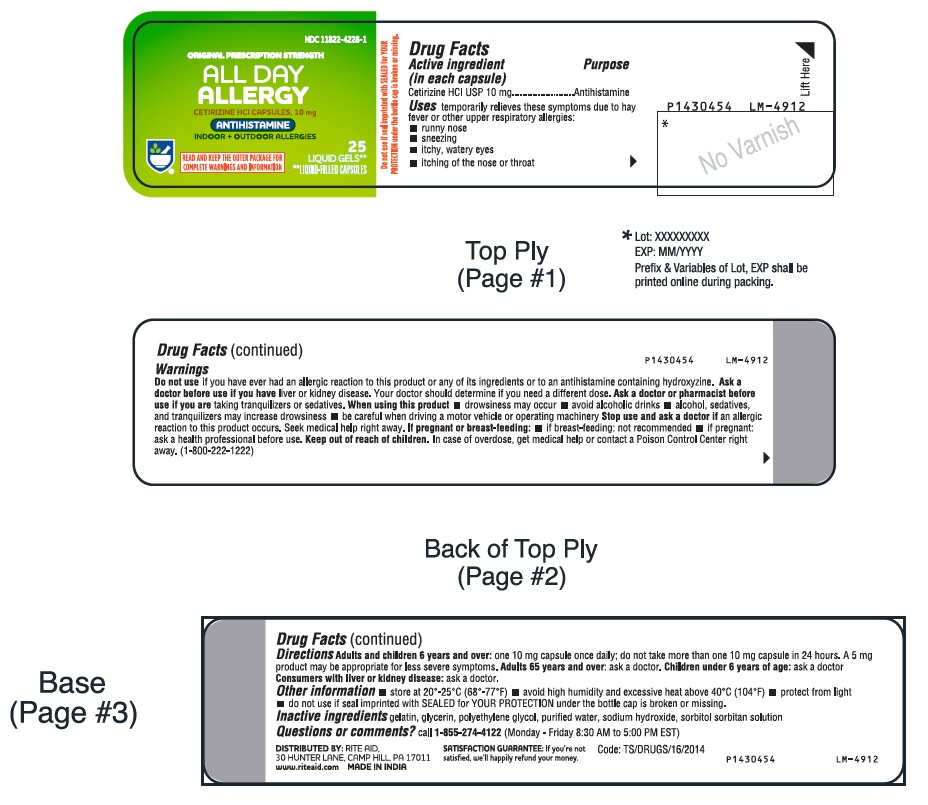
- Active ingredient (in each capsule)
- Purpose
- Uses
- Warnings
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- When using this product
- Stop use and ask a doctor if
- If pregnant or breast-feeding:
- Keep out of reach of children.
-
Directions
adults and children 6 years and over
one 10 mg capsule once daily; do not take more than one 10 mg capsule in 24 hours. A 5 mg product may be appropriate for less severe symptoms.
adults 65 years and over
ask a doctor
children under 6 years of age
ask a doctor
consumers with liver or kidney disease
ask a doctor
- Other information
- Inactive ingredients
- Questions or comments?
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 10 mg (25's Capsule Container Label)
-
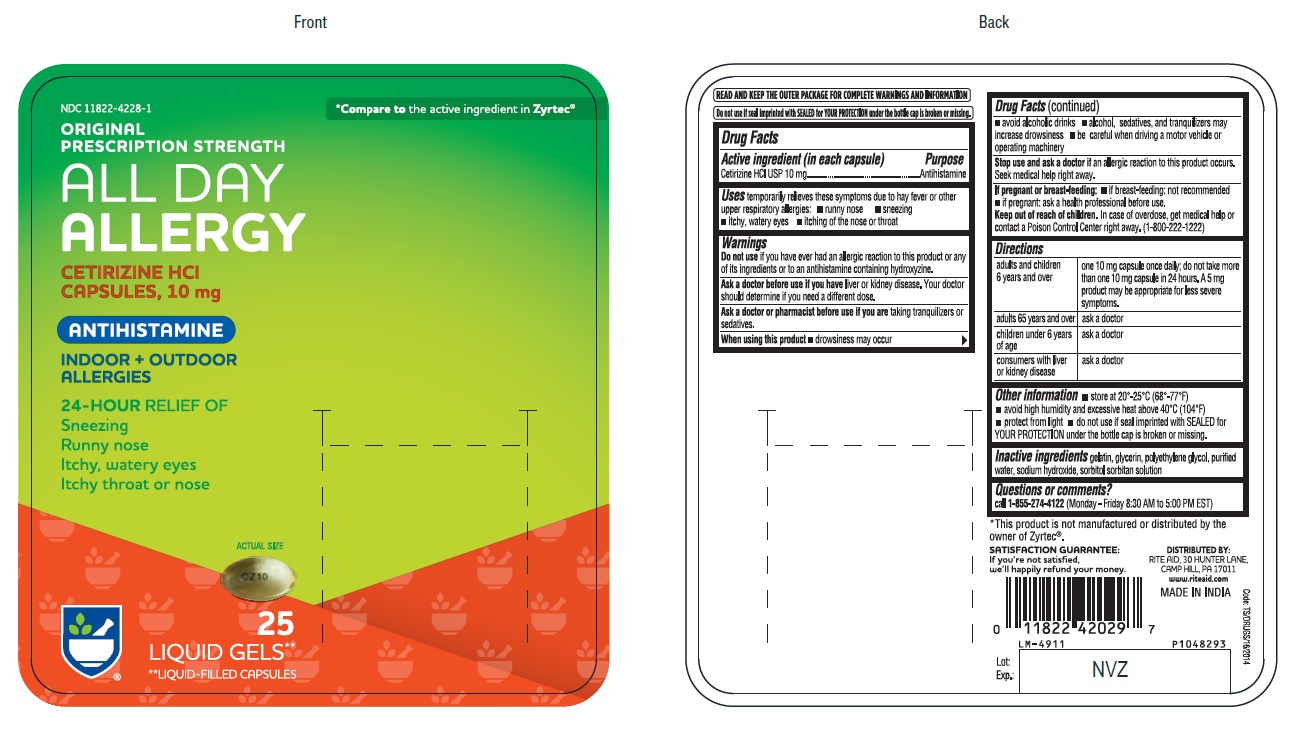
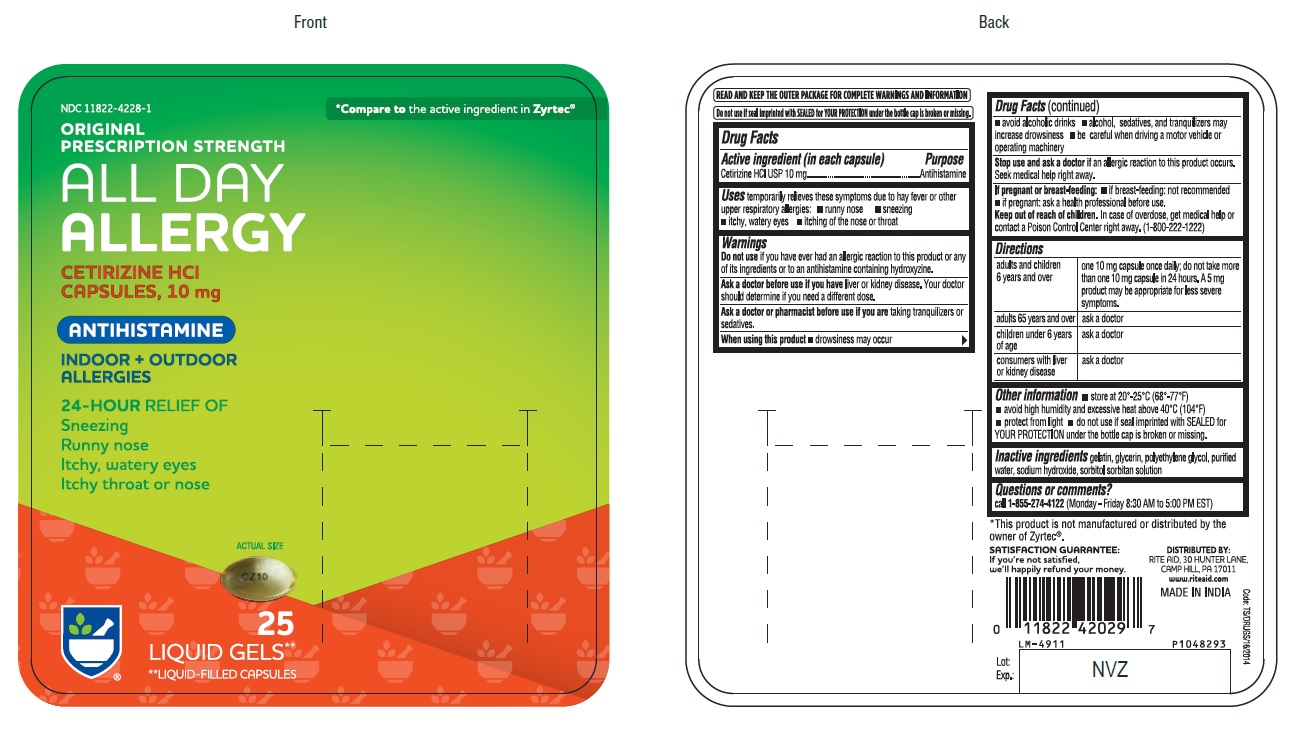
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL -10 mg (25's Capsule Container Carton Label)
NDC 11822-4228-1
*Compare to the active ingredient in Zyrtec®
ORIGINAL
PRESCRIPTION STRENGTH
ALL DAY
ALLERGY
CETIRIZINE HCl
CAPSULES, 10 mg
ANTIHISTAMINE
INDOOR +OUTDOOR
ALLERGIES
24-HOUR RELIEF OFSneezing
Runny nose
Itchy, watery eyes
Itchy throat or nose
ACTUAL SIZE
CZ10
25
LIQUID GELS**
**LIQUID-FILLED CAPSULES
-
INGREDIENTS AND APPEARANCE
CETIRIZINE HYDROCHLORIDE (ALLERGY)
cetirizine hydrochloride capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11822-4228 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength GELATIN, UNSPECIFIED (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) SORBITOL (UNII: 506T60A25R) Product Characteristics Color YELLOW (Clear colourless to pale yellow viscous liquid) Score no score Shape OVAL Size 13mm Flavor Imprint Code CZ10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11822-4228-1 1 in 1 CARTON 06/24/2022 1 25 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:11822-4228-2 1 in 1 CARTON 06/24/2022 2 40 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209107 06/24/2022 Labeler - RITE AID CORPORATION (014578892) Registrant - Aurohealth LLC (078728447) Establishment Name Address ID/FEI Business Operations APL HEALTHCARE LIMITED 650844777 ANALYSIS(11822-4228) , MANUFACTURE(11822-4228) Establishment Name Address ID/FEI Business Operations Aurobindo Pharma Limited 650381903 ANALYSIS(11822-4228) , MANUFACTURE(11822-4228)