Label: SWEET BALM- peppermint oil stick
-
Contains inactivated NDC Code(s)
NDC Code(s): 82690-302-01 - Packager: isamogu Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 2, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Warnings
- Warnings
- Warnings
- Warnings
- Directions
-
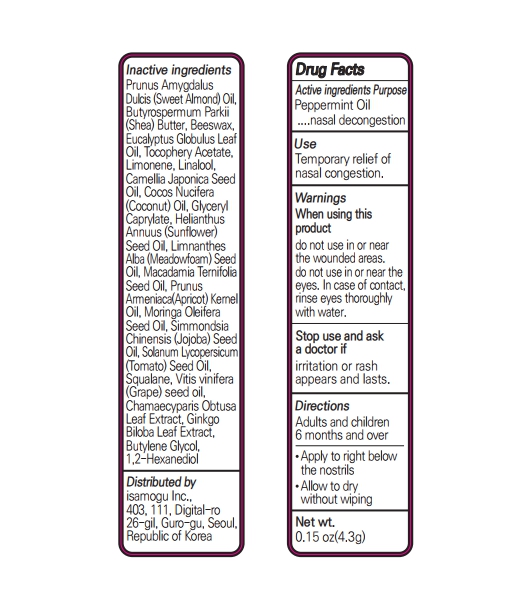
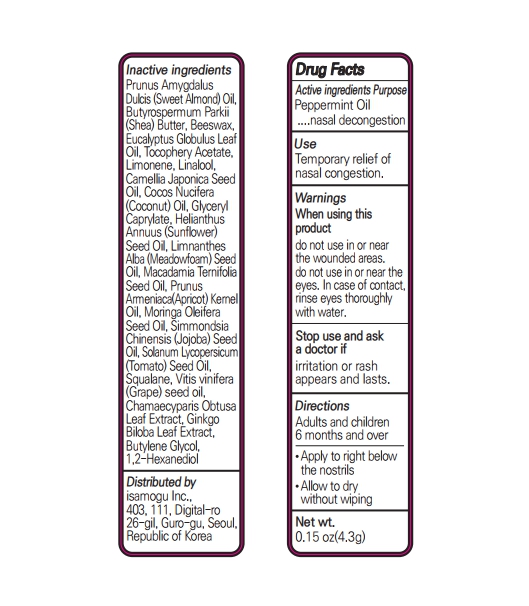
Inactive Ingredients
Prunus AmygdalusDulcis (Sweet Almond) Oil, Butyrospermum Parkii (Shea) Butter, Beeswax, Eucalyptus Globulus Leaf Oil, Tocophery Acetate, Limonene, Linalool, Camellia Japonica Seed Oil, Cocos Nucifera (Coconut) Oil, Glyceryl Caprylate, Helianthus Annuus (Sunflower) Seed Oil, Limnanthes Alba (Meadowfoam) Seed Oil, Macadamia Ternifolia Seed Oil, Prunus Armeniaca(Apricot) Kernel Oil, Moringa Oleifera Seed Oil, Simmondsia Chinensis (Jojoba) Seed Oil, Solanum Lycopersicum (Tomato) Seed Oil, Squalane, Vitis vinifera (Grape) seed oil, Chamaecyparis Obtusa Leaf Extract, Ginkgo Biloba Leaf Extract, Butylene Glycol, 1,2-Hexanediol
- Sweet Balm Label
-
INGREDIENTS AND APPEARANCE
SWEET BALM
peppermint oil stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82690-302 Route of Administration TOPICAL, RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PEPPERMINT OIL (UNII: AV092KU4JH) (PEPPERMINT - UNII:V95R5KMY2B) PEPPERMINT OIL 0.65 g in 100 g Inactive Ingredients Ingredient Name Strength CHAMAECYPARIS OBTUSA LEAF (UNII: 7OL154J5XB) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) LINALOOL, (+/-)- (UNII: D81QY6I88E) APRICOT KERNEL OIL (UNII: 54JB35T06A) ALMOND OIL (UNII: 66YXD4DKO9) SHEA BUTTER (UNII: K49155WL9Y) YELLOW WAX (UNII: 2ZA36H0S2V) EUCALYPTUS OIL (UNII: 2R04ONI662) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) COCONUT OIL (UNII: Q9L0O73W7L) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) SUNFLOWER OIL (UNII: 3W1JG795YI) MEADOWFOAM SEED OIL (UNII: 412ZHA4T4Y) MACADAMIA OIL (UNII: 515610SU8C) MORINGA OLEIFERA SEED OIL (UNII: REM6A5QMC0) SQUALANE (UNII: GW89575KF9) GRAPE SEED OIL (UNII: 930MLC8XGG) GINKGO (UNII: 19FUJ2C58T) JOJOBA OIL (UNII: 724GKU717M) TOMATO SEED OIL (UNII: 7N87T9C06T) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) LIMONENE, (+)- (UNII: GFD7C86Q1W) CAMELLIA JAPONICA SEED OIL (UNII: U37N0S910T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82690-302-01 4.3 g in 1 TUBE; Type 0: Not a Combination Product 05/03/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 05/03/2022 Labeler - isamogu Inc. (695695834) Registrant - isamogu Inc. (695695834) Establishment Name Address ID/FEI Business Operations Isamogu Inc. 695695834 manufacture(82690-302)